A kind of fumarate of rifamycin-quinazinone double-target molecule and preparation method thereof
A technology of rifamycin and fumarate, which is applied in the direction of organic chemistry and can solve the problems of undisclosed fumarate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
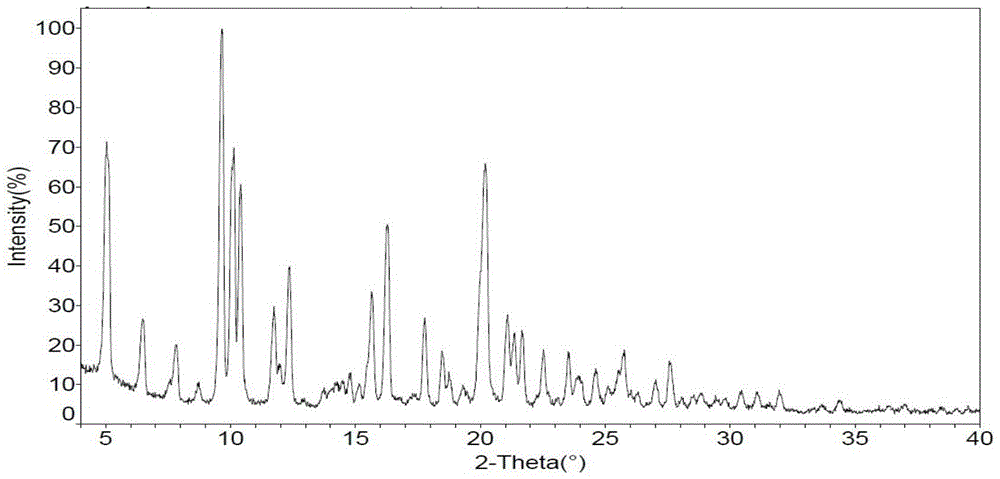
[0030] Example 1 Determination of the approximate solubility of crystal form I of the rifamycin-quinazinone dual target molecule at room temperature
[0031] Experimental procedure: Weigh about 2 mg of rifamycin-quinazinone dual target molecule ((R)-3-[(4-{1-[1-(3-carboxy-1-cyclopropyl-7-fluoro -9-Methyl-4-oxo-4H-quinazine-8-yl)-pyrrolidin-3-yl-cyclopropyl]-methylamino)-piperidin-1-ylimino)-methylene ]-Rifamycin SV) crystal form Ⅰ (the X-ray powder diffraction pattern is as figure 1 (Shown) into a 1.5mL glass bottle, and gradually add the selected solvent (see Table 1) at room temperature until the compound is completely dissolved. The experiment was carried out at room temperature by manual gradual dilution and visual inspection, and finally recorded the total solvent added Volume, calculate the approximate solubility of the compound, see Table 1.
[0032] The experimental results show that the crystal form I of the rifamycin-quinazinone dual target molecule has relatively good s...
Embodiment 2
[0035] Example 2 Screening of Fumarate of Rifamycin-Quazinone Dual Target Molecule in Tetrahydrofuran System
[0036] Experimental method: Using tetrahydrofuran as a solvent, 12 acids (see Table 2) were selected for the salt formation reaction with the crystal form I of the rifamycin-quinazinone dual target molecule. Weigh 150 mg of the rifamycin-quinazinone dual target molecule crystal form I to 12 glass sample vials, add 2.5 mL of tetrahydrofuran to each to dissolve the sample, according to the free state of the rifamycin-quinazinone dual target molecule: The molar ratio of acid or base=1:1 dissolve the selected acid in a certain dose of tetrahydrofuran and slowly add it to the sample bottle, and continue stirring at room temperature for 24 hours. The crystal form I of the rifamycin-quinazinone dual target molecule was also treated in the same way and placed in parallel as a reference. After 24 hours, all the obtained solutions or suspensions were evaporated to dryness at room...
Embodiment 3
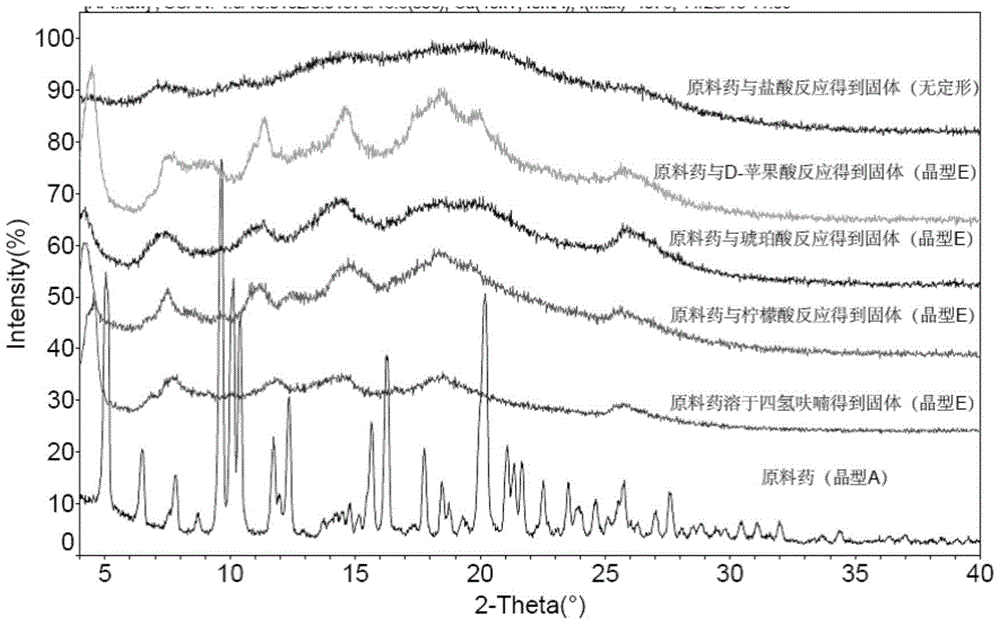
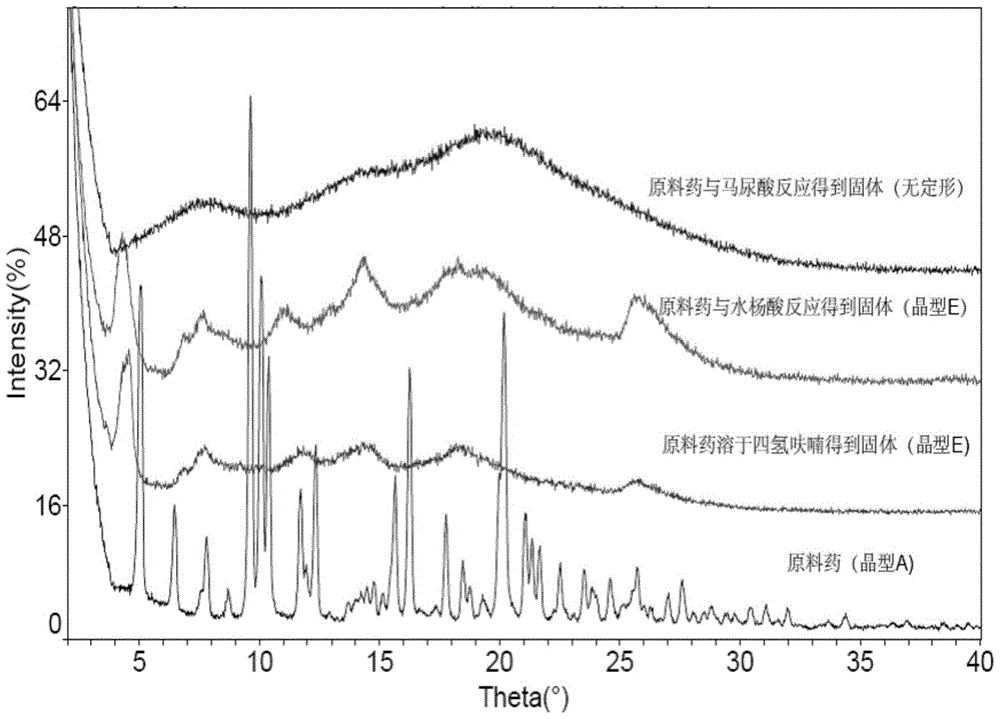
[0042] Example 3 Screening of crystal form of fumarate of rifamycin-quinazinone dual target molecule
[0043] Experimental procedure: In order to obtain the fumarate of the rifamycin-quinazinone dual target molecule with better crystallinity, the fumarate of the rifamycin-quinazinone dual target molecule was subjected to suspension vibration. For the shaking method, four solvent systems were selected (see Table 3). The specific operation method is as follows: Weigh approximately 10 mg of fumarate of the rifamycin-quinazinone dual target molecule into a 1.5 mL plastic centrifuge tube, add 300 μL of solvent to obtain a suspension solution, seal the centrifuge tube and place it at 25°C Shake continuously for 24h in the dark. After 24h, the solid in the suspension was separated by centrifugation and subjected to X-ray powder diffraction analysis (see Table 3, Figure 5 ).
[0044] According to the results of X-ray powder diffraction, the fumarate and rifamycin-quinoline of the rifamyc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap