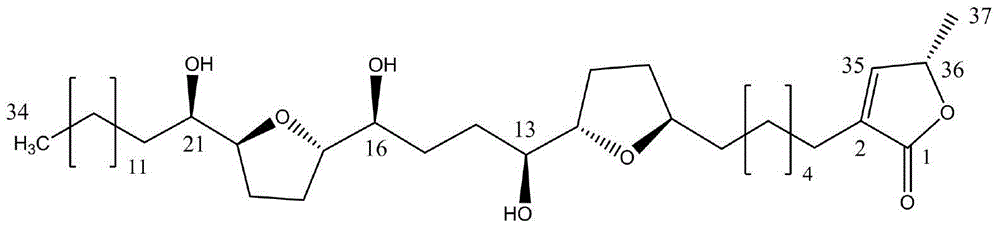
Application of annosquatin A in preparation of pesticide preparation
A technology for pesticide preparation and methanol is applied in the field of preparation of succinyl lactone compounds, which can solve the problems of complex method, low yield, and no known preparation method for annoskotin first, and achieves low environmental pollution and good quality. The effect of poisoning activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 2kg of custard apple seeds, crush them, and percolate the coarse powder twice with 5 times the amount of ethyl acetate, combine the percolation liquid, recover under reduced pressure below 60°C to obtain the concentrated liquid, let it stand overnight, and centrifuge at 3000r / min to obtain the obtained product The upper layer (123g), mixed with 120g of silica gel, was subjected to 500g silica gel column chromatography, and was eluted with petroleum ether-ethyl acetate gradient, the ratios were 10:1, 5:1, 2:1, 1:1, 0:1 , 10 fractions were collected for each ratio, each fraction was 500 mL, tracked by thin-layer chromatography, 33-40 fractions were combined, concentrated and dried under reduced pressure at less than 60°C. Recrystallize with 5 times the amount of petroleum ether-ethyl acetate (5:1) to obtain 0.2 g of refined anoxactine A. Its molecular weight was determined to be 622.4 by 1H-NMR data and MS, and it was determined to be annulotide anorostecidine A.
Embodiment 2
[0025] Take 1.8kg of custard apple seeds, crush them, and pass the coarse powder through 20 meshes, and perform supercritical carbon dioxide extraction under the conditions of extraction temperature 35°C, extraction pressure 30Mpa, extraction time 3 hours and entrainer ethanol consumption 180mL. The extract was obtained, which was recovered under reduced pressure below 60°C to obtain 98 g of concentrated liquid. After mixing 100g of silica gel, carry out 500g of silica gel column chromatography, and use chloroform-methanol gradient elution, the ratio is 100:1, 50:1, 25:1, 10:1, each ratio collects 10 fractions, each fraction is 500mL , traced by thin layer chromatography, combined 15-21 fractions, concentrated and dried under reduced pressure at less than 60°C. Recrystallize with 5 times the amount of chloroform-methanol (50:1) to obtain 0.23 g of refined anoxetine A. Its molecular weight was determined to be 622.4 by 1H-NMR data and MS, and it was determined to be annulotide...
Embodiment 3
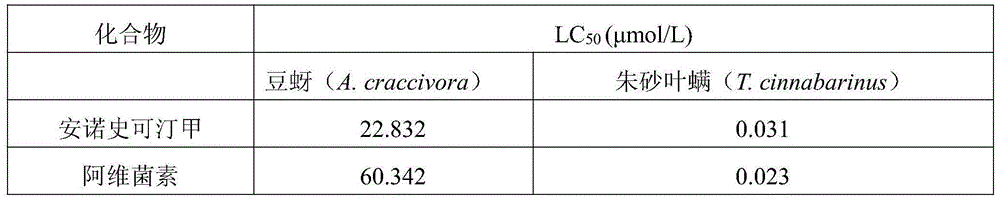
[0026] The biological activity of embodiment 3 Anoxetine A
[0027] 1. Determination of the contact effect on bean aphid:
[0028] Use a micro-dropper to drip 10% distilled acetone aqueous solution containing different concentrations of anoxactin A on the abdomen and back of the bean aphid, and the amount of dripping per insect is 0.05 μL. After the dripping was completed, each was put into a biochemical incubator for cultivation. After 24 hours, the death situation was checked and the death rate was calculated. Immersion with 10% acetone distilled water was used as a control. The mortality rate of the control group was less than 10% for effective determination, and the mortality rate of the treatment group was corrected by Abbott's formula. Measured 3 times in parallel. The test results were analyzed using SPSS 13.0 to calculate the lethal concentration LC 50 .
[0029] 2. Determination of contact killing effect on Tetranychus cinnabarinus:
[0030] Paste the double-si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com