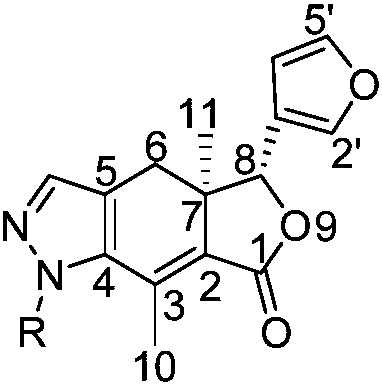
N-(substituted phenyl) pyrazolo arketone derivatives, preparation method and application thereof
A technology of pyrazoloalkone and its derivatives, which is applied in the field of organic chemistry, can solve the problems of synthesis of alkone derivatives, few reports on insecticidal activity, poor contact activity, etc., and achieve simple and feasible synthetic route and good antibacterial activity , good growth inhibitory and poisonous activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of 4-carbonyl-5-(dimethylaminomethylene) alkone (c)
[0028]Dissolve 200mg of chromium trioxide and 316.4mg of pyridine with anhydrous dichloromethane, then add 1.8g of tert-butyl hydroperoxide and stir for 3 minutes, finally add 464mg of alkone, react at room temperature, TLC detection, the reaction is complete Afterwards, the compound alkone ketone was separated by column chromatography under reduced pressure; 246 mg of the compound alkone ketone was dissolved in an appropriate amount of toluene, and then 143 mg of N,N-dimethylformamide dimethyl acetal was added at 110 ° C. The reaction was detected by TLC. After the reaction was complete, it was concentrated under reduced pressure and separated by column chromatography to obtain 4-carbonyl-5-(dimethylaminomethylene) alkone (c).
[0029] The physical and chemical properties of 4-carbonyl-5-(dimethylaminomethylene) arketone (c):
[0030] 1), orange-yellow solid, melting point 66-68°C.
[0031] 2),...
Embodiment 2
[0035] Example 2 N-phenylpyrazolo alkone derivatives (compound 1)
[0036] Dissolve 72.3 mg of 4-carbonyl-5-(dimethylaminomethylene) arketone (c) and 31.1 mg of phenylhydrazine hydrochloride in absolute ethanol, heat to reflux, and track and detect with TLC. After the reaction is completed, depressurize After concentrating and spinning the ethanol to dryness, adding an appropriate amount of dichloromethane to dissolve, and then separating by thin-layer chromatography to obtain pure compound 1 with a yield of 83%.
[0037] The physical and chemical properties of compound 1 are as follows:
[0038] 1), orange-yellow solid, melting point 85-88°C.
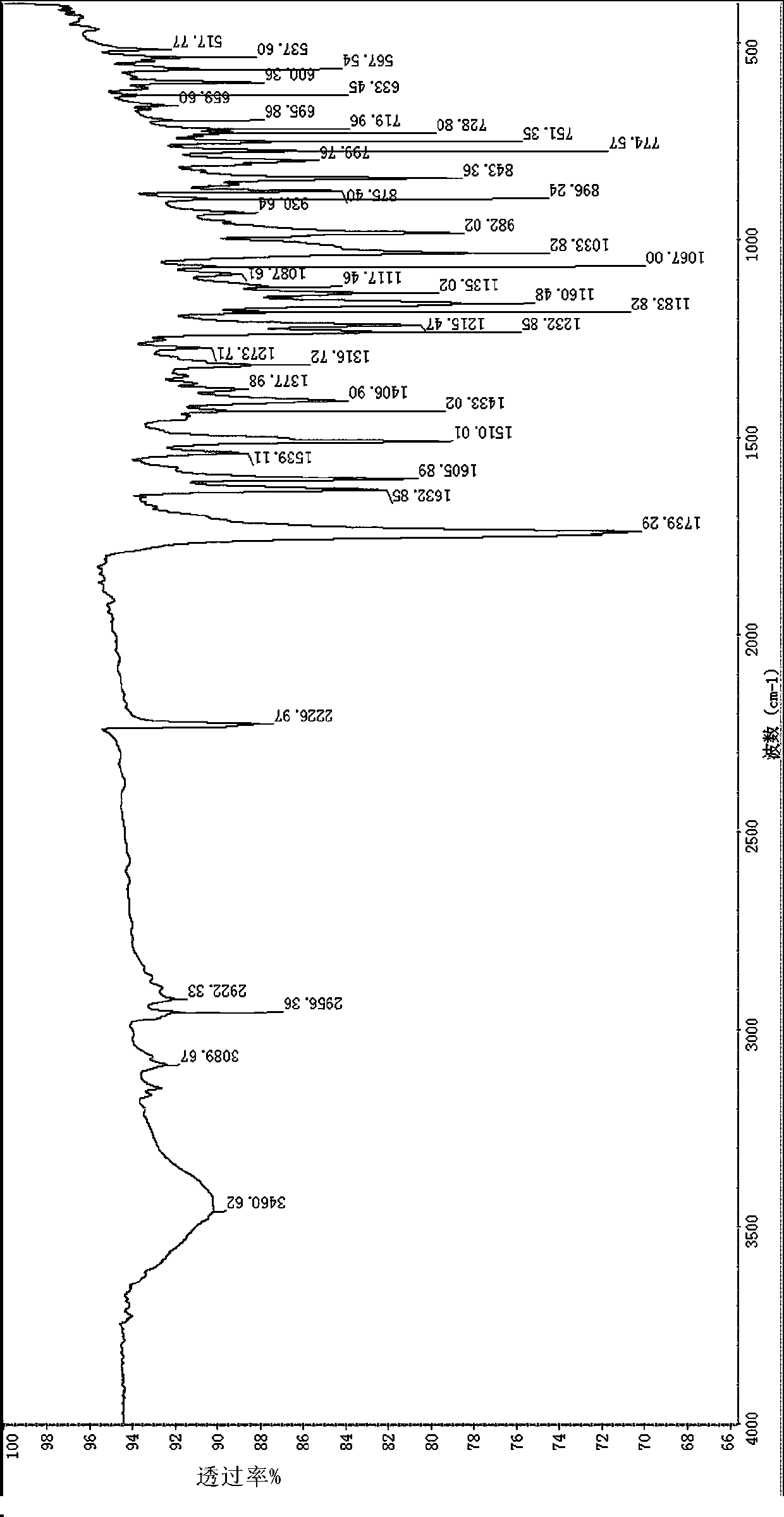
[0039] 2), the infrared spectrogram (IR) feature of this compound:
[0040] Using potassium bromide tablet method: 2961, 2923cm -1 Stretching vibration absorption for saturated hydrocarbons, 1736cm -1 Stretching vibration absorption for lactone carbonyl, 1024cm -1 It is C-O-C stretching vibration absorption.
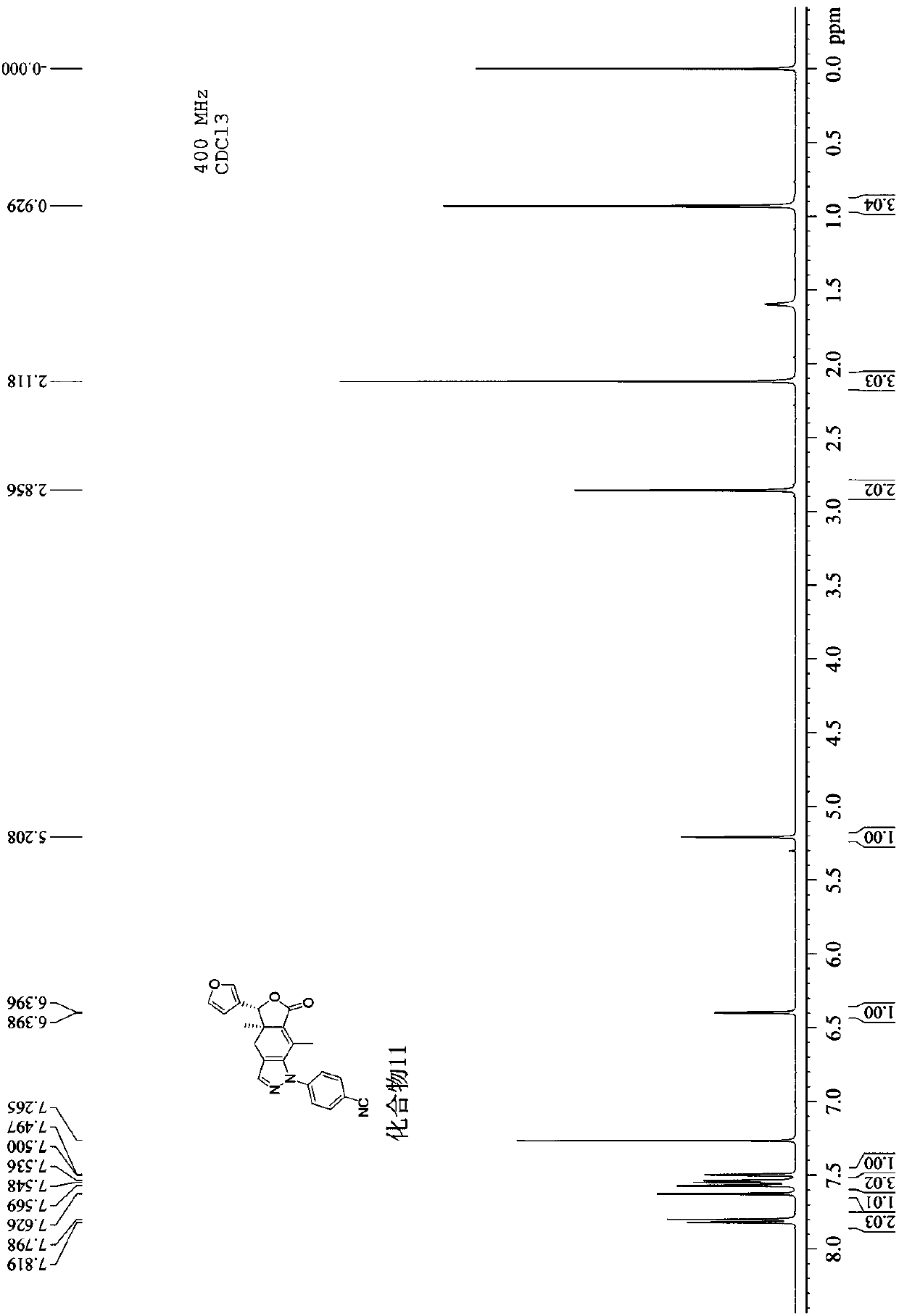
[0041] 3), the nucl...
Embodiment 3
[0043] Example 3 N-(2-fluoro-phenyl) pyrazolo arketone derivatives (compound 2)
[0044] Adopt the method described in embodiment 2 to synthesize compound 2, the physicochemical property of compound 2 is as follows:
[0045] 1) Light yellow solid, melting point 204-206°C.
[0046] 2), the infrared spectrogram (IR) feature of this compound:
[0047] Using potassium bromide tablet method: 3132cm -1 Stretching vibration absorption for unsaturated hydrocarbons, 2962, 2925cm -1 Stretching vibration absorption for saturated hydrocarbons, 1741cm -1 Stretching vibration absorption for lactone carbonyl, 1041cm -1 It is C-O-C stretching vibration absorption.
[0048] 3), the nuclear magnetic resonance spectrum of this compound ( 1 H NMR, 400MHz) features:
[0049] With deuterated chloroform as the solvent and TMS as the internal standard, the peaks are assigned as follows: δ: 7.60-7.67 (m, 2H, -Ph and-CH=N-), 7.53 (s, 1H, H-2′), 7.45-7.52(m,2H,-Ph andH-5′),7.31-7.35(m,1H,-Ph),7....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com