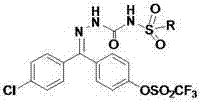
Benzophenone hydrazone sulfonylurea compound and its preparation method and use
A technology for benzophenone hydrazone sulfonylurea and compound, which is applied in the field of new benzophenone hydrazone sulfonylurea compounds and achieves the effects of excellent application prospect, high product purity and strong poisoning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of N-methylsulfonyl-[(4-chlorophenyl)(4-trifluoromethylsulfonylphenyl)-keto]semicarbazone (6a)
[0025] Synthesis of raw material benzophenone hydrazone precursor (5): Dissolve 0.1mmol of phenol in 100mL of dry dichloroethane, quickly weigh and add 1.5mmol of anhydrous aluminum trichloride, heat to about 70°C in an oil bath, 0.1 mmol of 4-chlorobenzoyl chloride was added dropwise to the reaction liquid, and stirring was continued for 2 hours after the dropwise addition was completed. Cooled, poured into 100mL of ice water, extracted three times with dichloromethane, separated the organic phase, dried with anhydrous sodium sulfate, evaporated the solvent under reduced pressure, and recrystallized with anhydrous methanol to obtain 4-chloro-4- Hydroxybenzophenones (3). Dissolve 0.05mmol of 4-chloro-4-hydroxybenzophenone in 50mL of dry dichloromethane, drop into 0.05mL of pyridine, control the temperature at 0°C, add 0.06mmol of trifluoromethanesulfonic anhydride...
Embodiment 2
[0034] Synthesis of N-naphthalenesulfonyl-[(4-chlorophenyl)(4-trifluoromethylsulfonylphenyl)-keto]semicarbazone (6b)
[0035] The experimental procedure is the same as in Example 1, except that methyl is replaced by naphthyl. The detection data of the product obtained from the reaction are as follows: Yield: 55%; Melting point: 198-200°C; 1 H NMR (400MHz, DMSO-d 6 )δ:7.21(d,J=8.4Hz,1H,Ar-H),7.27(d,J=8.0Hz,1H,Ar-H),7.39-7.47(m,4H,Ar-H),7.59( d,J=8.4Hz,2H,Ar-H),7.65-7.74(m,3H,Ar-H),7.91(d,J=8.8Hz,1H,Ar-H),8.05(d,J=7.2 Hz,1H,Ar-H),8.14(d,J=8.8Hz,1H,Ar-H),8.23(d,J=8.0Hz,1H,Ar-H),9.55(s,1H,-CONHSO 2 -);MS-ESI m / z:612.3[M+H] + .
Embodiment 3
[0037] Synthesis of N-p-fluorobenzoyl-[(4-chlorophenyl)(4-trifluoromethylsulfonylphenyl)-one]semicarbazone (6c)
[0038] The experimental procedure is the same as in Example 1, except that p-fluorophenyl is used instead of methyl. The detection data of the product obtained from the reaction are as follows: Yield: 48%; Melting point: 209-210°C; 1 H NMR (400MHz, DMSO-d 6 )δ:7.21(d,J=8.4Hz,1H,Ar-H),7.29(d,J=8.4Hz,1H,Ar-H),7.37-7.50(m,7H,Ar-H),7.59( d,J=8.4Hz,1H,Ar-H),7.66(d,J=8.4Hz,1H,Ar-H),7.91-7.97(m,1H,Ar-H),9.24(s,1H,- CONHSO 2 -);MS-ESI m / z:579.9[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com