Coupling oligoarginine and 2 site substituted endomorphin-1 analogue as well as synthetic method and application thereof
A technology of oligoarginine and endomorphin, which is applied to the preparation method of peptides, drug combinations, chemical instruments and methods, etc., to achieve good stability and enhance the permeability of the blood-brain barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
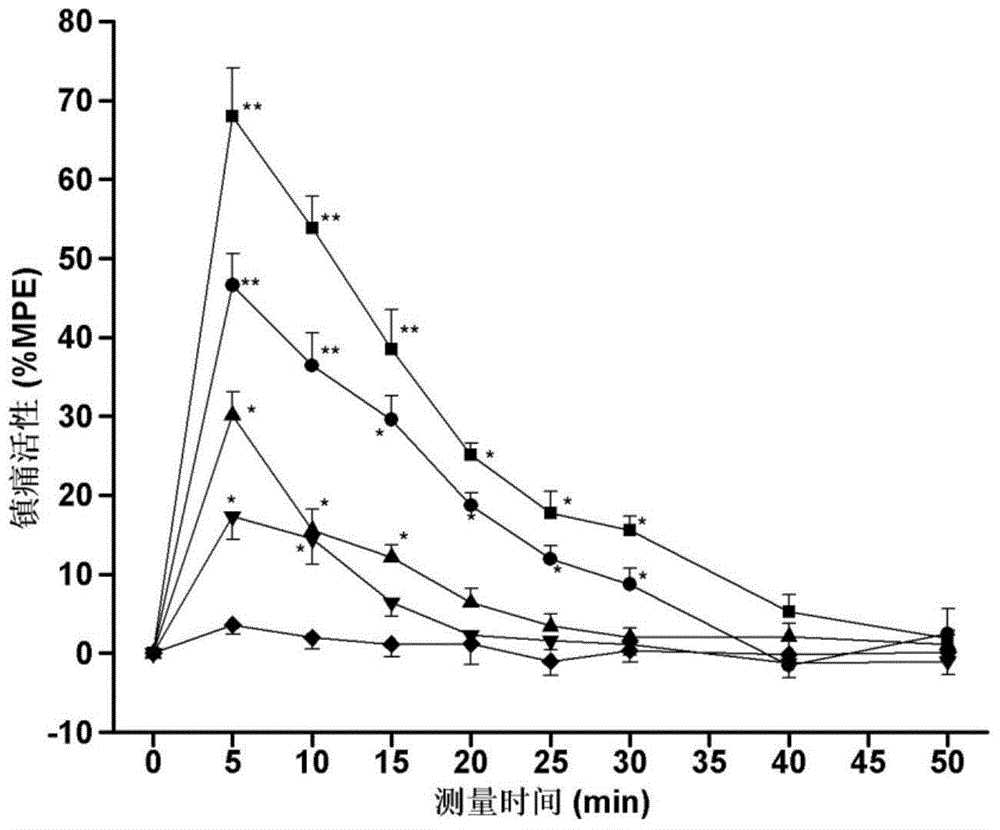
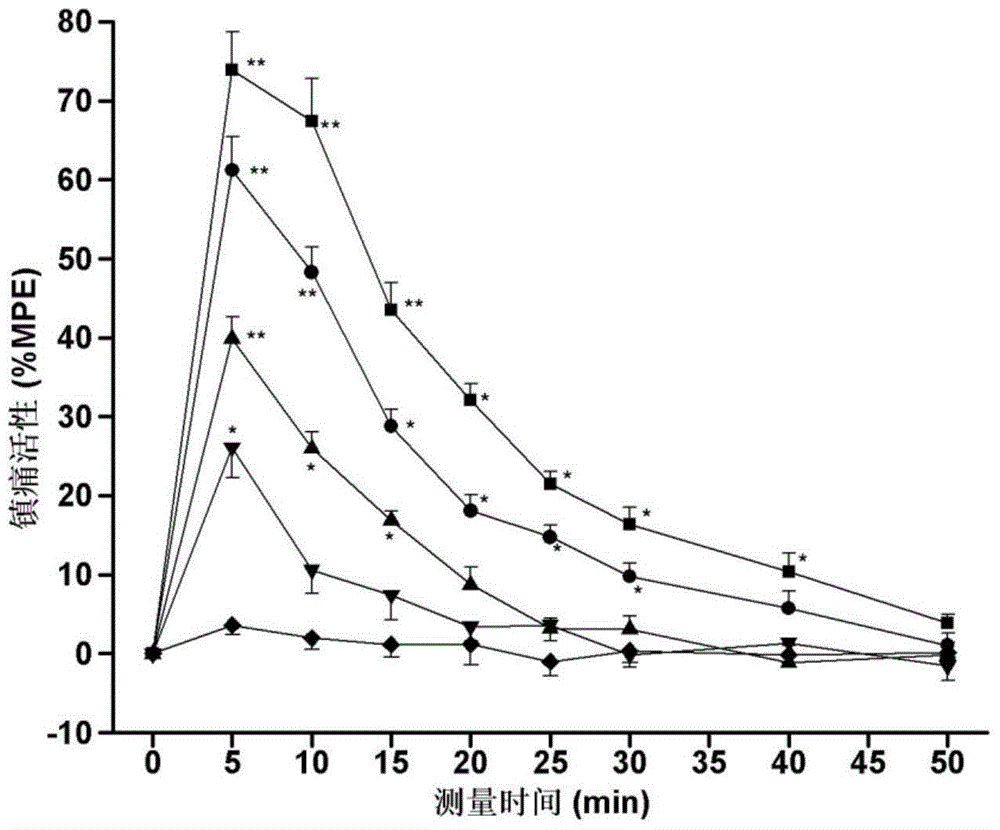
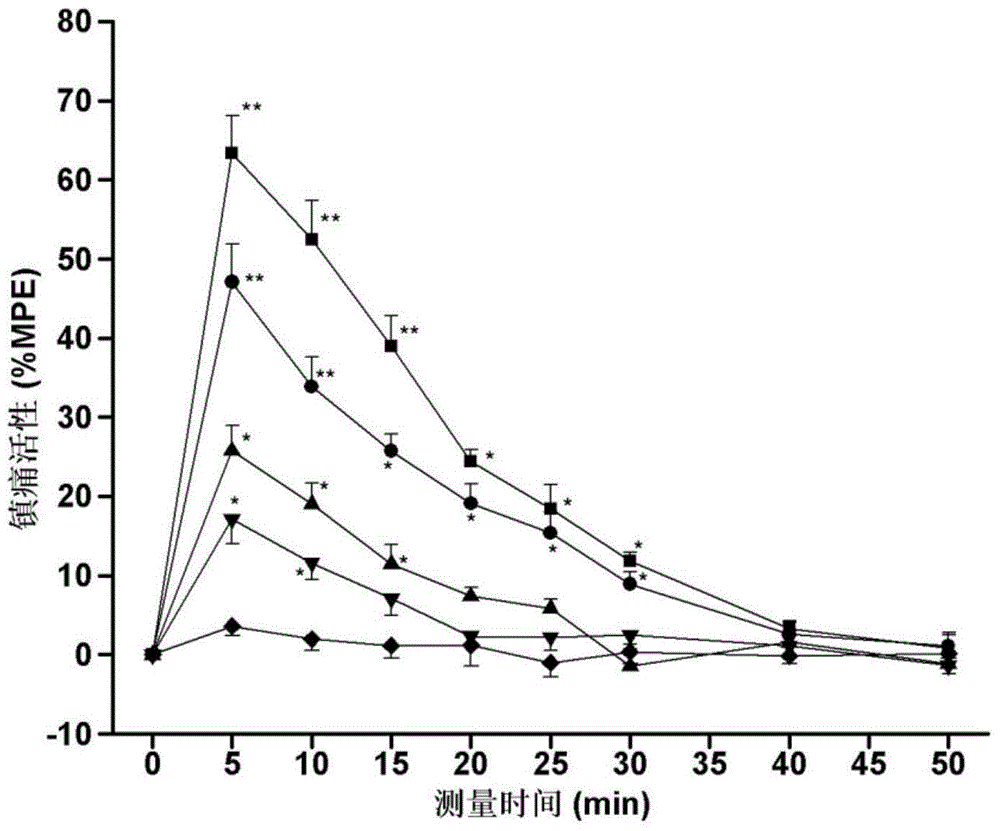
[0034] Specific embodiment 1: The structure of the endomorphin-1 analogue coupled with oligoarginine and 2-position substitution in this embodiment is as follows:
[0035]
[0036] Wherein AA is β-Pro, D-Ala or Sar, n is 2 or 5.
[0037]While the endomorphin-1 analog of this embodiment retains opioid affinity and agonist activity, it has higher biological stability and analgesic activity of peripheral administration than the parent endomorphin-1, and reduces gastrointestinal Road side effects and other advantages. Experiments have confirmed the importance of oligoarginine and unnatural amino acid combination modification method for improving drug utilization and efficacy, which provides broad prospects for the development of new neuropeptide drugs with medical value.
specific Embodiment approach 2
[0038] Specific embodiment 2: The synthesis method of coupling oligoarginine and 2-substituted endomorphin-1 analog in this embodiment is carried out according to the following steps:
[0039] 1. Pretreatment of "Fmoc"-protected Wang resin: put 1.2g of Fmoc-Arg(pbf)-Wang resin with one amino acid residue into the solid-phase synthesizer, add 8-12mL of dichloromethane and stir for 30min, depressurize Suction filtration until the solvent is drained;
[0040] 2. Remove the "Fmoc" protecting group: wash the swelled resin with DMF for 3 minutes, filter it under reduced pressure until it is drained, repeat 3 times;
[0041] Add 8-12 mL of piperidine / DMF deprotection solution with a concentration of 20% by volume to the resin, stir for 5 minutes and drain, repeat twice;
[0042] Then add 10-14 mL of piperidine / DMF deprotection solution with a concentration of 20% by volume, stir for 15 minutes, then drain the solvent, and finally wash with 8-12 mL of DMF to obtain a resin from which...
specific Embodiment approach 3
[0049] Embodiment 3: This embodiment differs from Embodiment 2 in that the molar weight of the amino acid protected by the "Fmoc" group in step 3 is 2.5 to 3 times the molar weight of the Fmoc-Arg(pbf)-Wang resin. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com