Multi-site modified enkephalin and neurotensin (8-13) coupled cyclic hybrid peptide and its synthesis method and application
A technology of neurotensin and its synthesis method, which is applied in the field of cyclized hybrid peptide and its synthesis, which can solve the problems of poor resistance to enzymolysis and poor anti-neuropathic pain effect, and achieve enhanced transport capacity and enhanced enzyme resistance solution ability, and the effect of improving biological stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
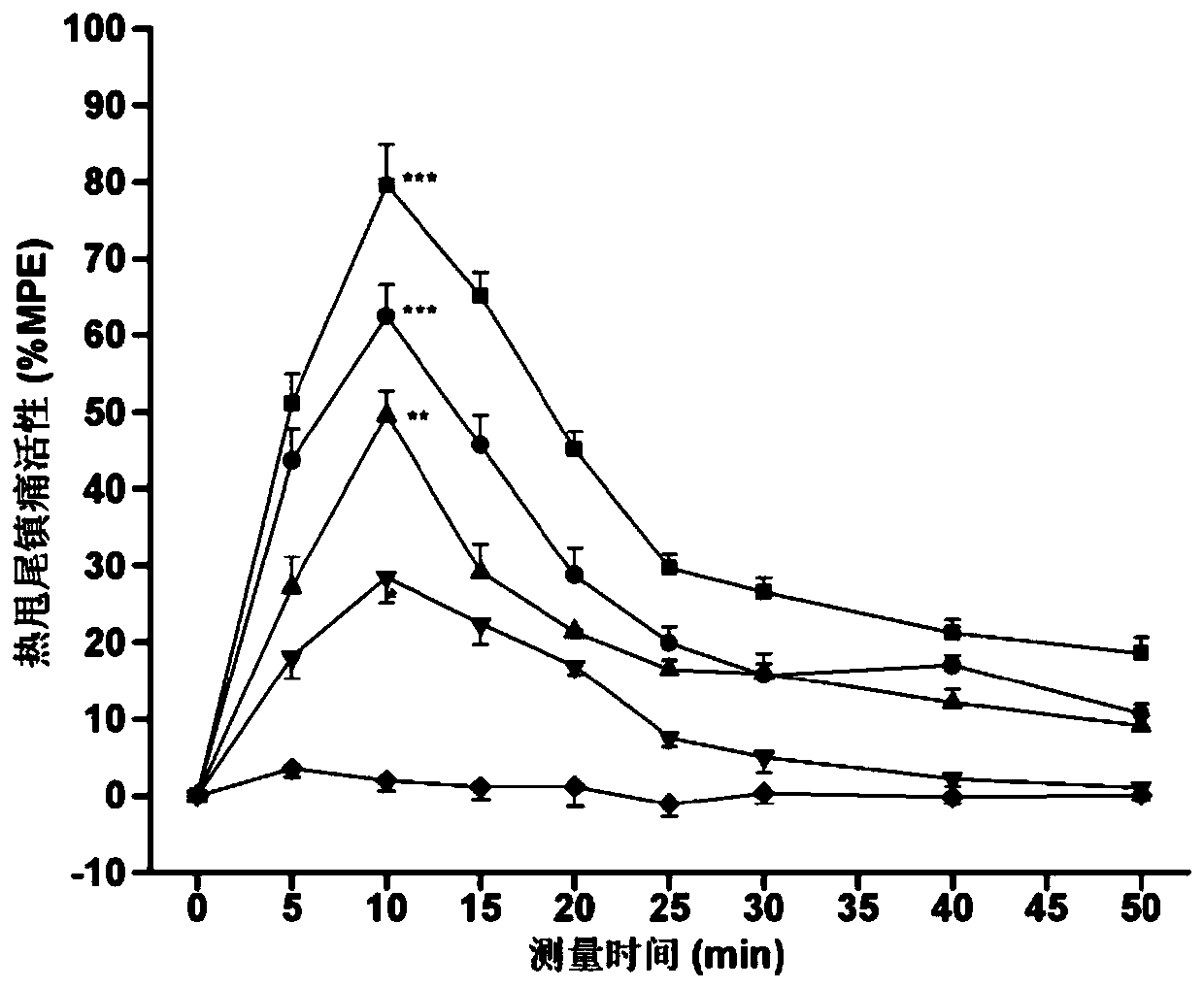
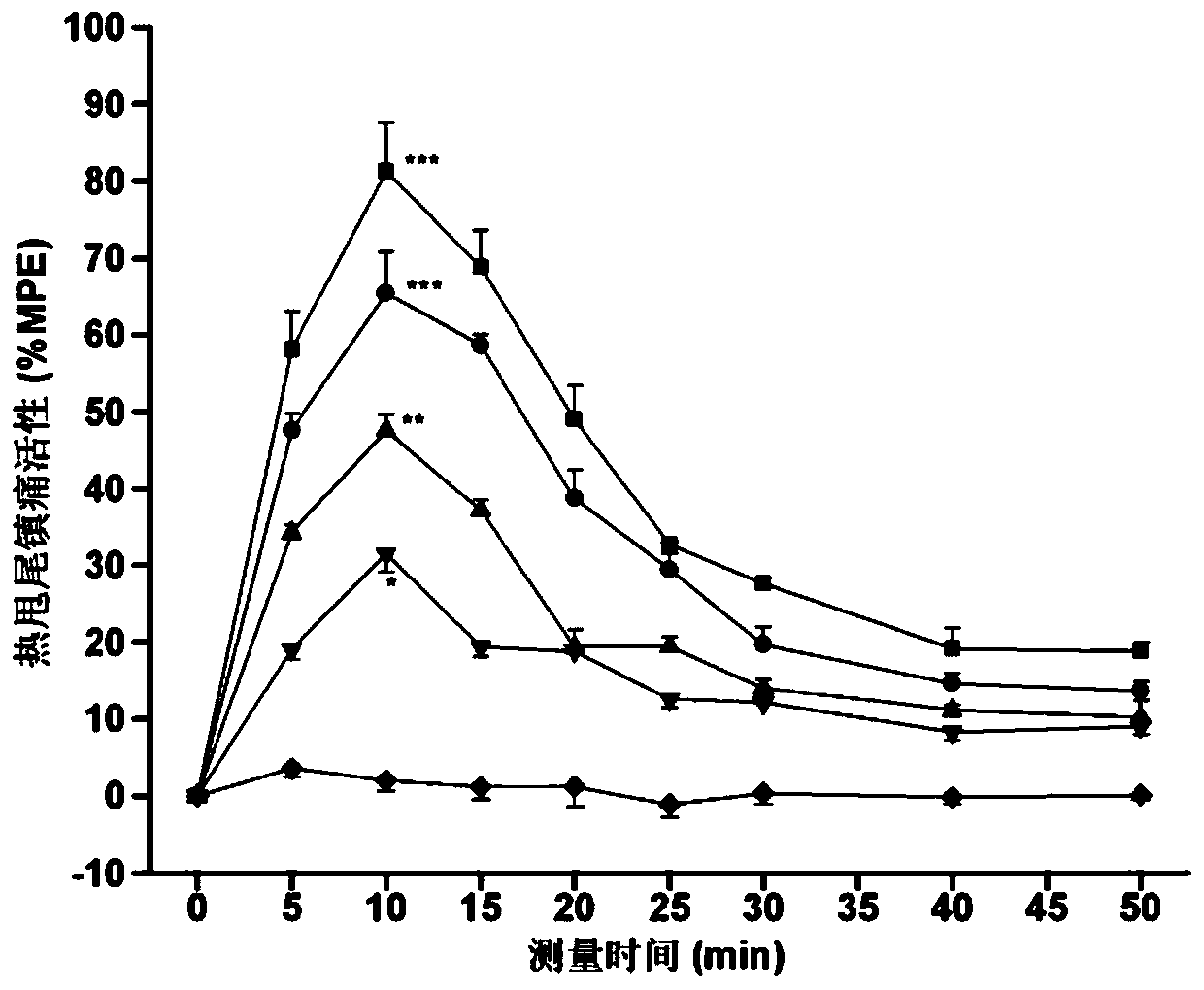
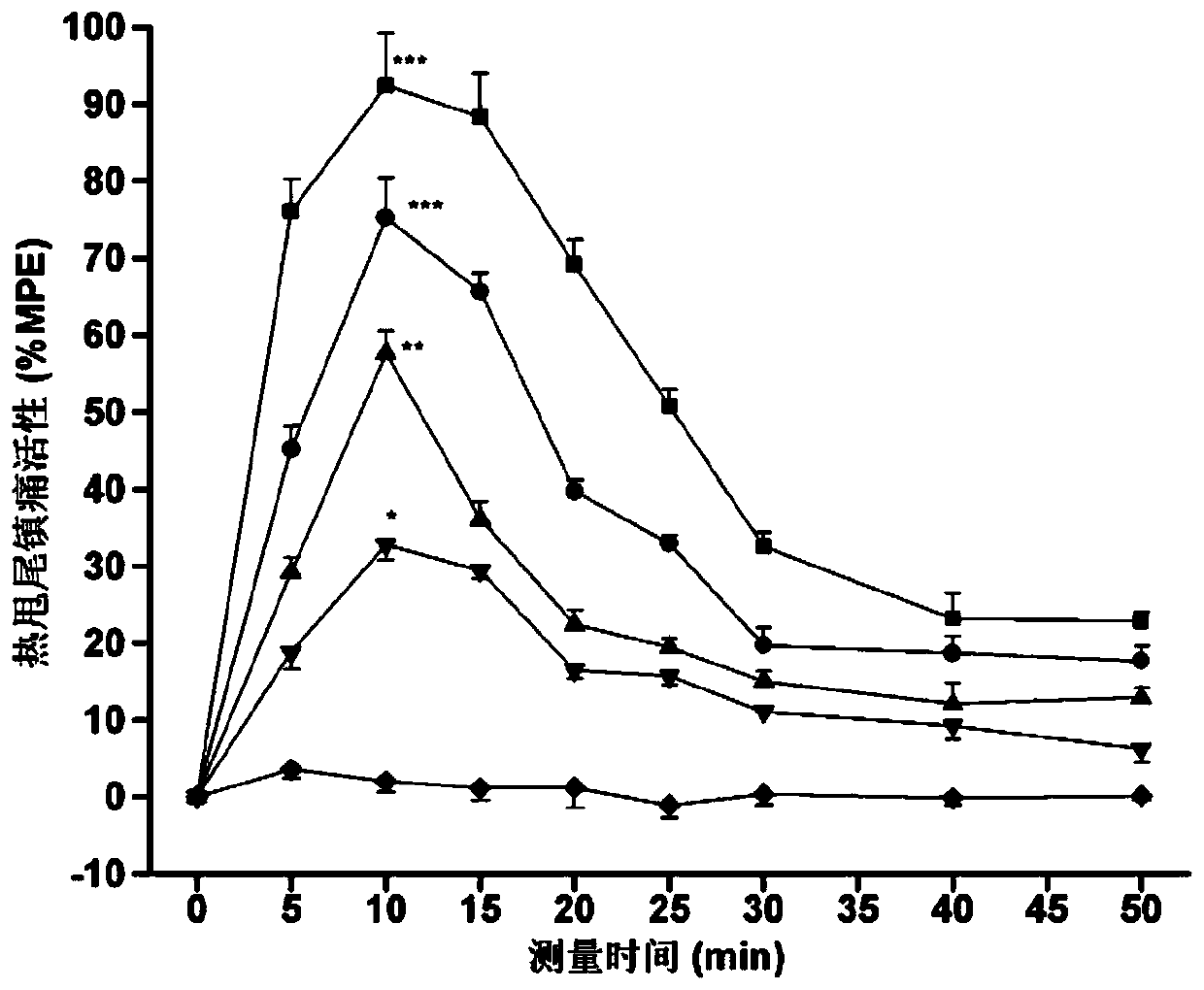
[0057] Specific embodiment 1: In this embodiment, the multi-site modified enkephalin and neurotensin (8-13) coupled cyclic hybrid peptides are hybrid peptide 1, hybrid peptide 2, and hybrid peptide 3 or hybrid peptide 4;
[0058] Wherein the amino acid sequence of hybrid peptide 1 is:
[0059] Dmt-Gly-c(Cys-NMePhe-Met-Cys)-Gly-NMeArg-Lys-Pro-Trp-Tle-Leu;
[0060] The amino acid sequence of hybrid peptide 2 is:
[0061] Dmt-Gly-c(Cys-NMePhe-Leu-Cys)-Gly-NMeArg-Lys-Pro-Trp-Tle-Leu;
[0062] The amino acid sequence of hybrid peptide 3 is:
[0063] Dmt-Gly-c(Cys-NMePhe-Met-Cys)-D-Ala-NMeArg-Lys-Pro-Trp-Tle-Leu;
[0064] The amino acid sequence of hybrid peptide 4 is:
[0065] Dmt-Gly-c(Cys-NMePhe-Leu-Cys)-D-Ala-NMeArg-Lys-Pro-Trp-Tle-Leu.
[0066] In this embodiment, the amino acid sequence "Tyr-Gly-Gly-Phe-Met / Leu" of methionine / leucine enkephalin is modified and cyclized to obtain "Dmt-Gly-c(Cys- NMePhe-Met-Cys)" and "Dmt-Gly-c (Cys-NMePhe-Leu-Cys)" amino acid residue seq...
specific Embodiment approach 2
[0068] Specific embodiment two: the synthetic method of the cyclized hybrid peptide of the multi-site modified enkephalin and neurotensin (8-13) coupling of the present embodiment, comprises the following steps:
[0069] 1. Pretreatment of Wang resin protected by "Fmoc": check the air tightness of the solid phase synthesizer, put the Fmoc-Leu-Wang resin with one amino acid residue into the synthesizer, add dichloromethane and stir for 30-40min, After the resin is fully soaked and swollen, filter the solvent under reduced pressure; the mass ratio of the Fmoc-Leu-Wang resin with one amino acid residue to the volume ratio of dichloromethane is 1g: (7-12)mL;
[0070] 2. Remove the "Fmoc" protecting group: wash the swelled resin with DMF for 3 to 5 minutes, dry it, repeat 3 to 5 times, and then add 20% to 25% vol. Piperidine / DMF deprotection solution, stirred for 5-10 minutes, drained, repeated 2-3 times, then added piperidine / DMF deprotection solution with volume percentage concentr...
specific Embodiment approach 3
[0078] Embodiment 3: This embodiment differs from Embodiment 2 in that the molar weight of the amino acid protected by the "Fmoc" group in step 3 is 2.5-3 times the molar weight of the Fmoc-Leu-Wang resin. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com