Novel pharmaceutical composition of 2-(4-isobutyl phenyl) propionic acid
A composition and drug technology, applied in the field of medicine, can solve the problems of content change, purity reduction, influence on preparation safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
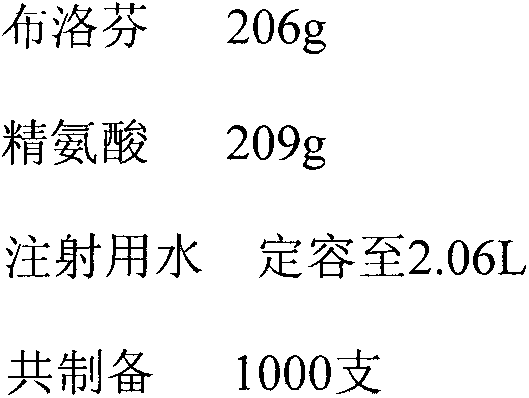
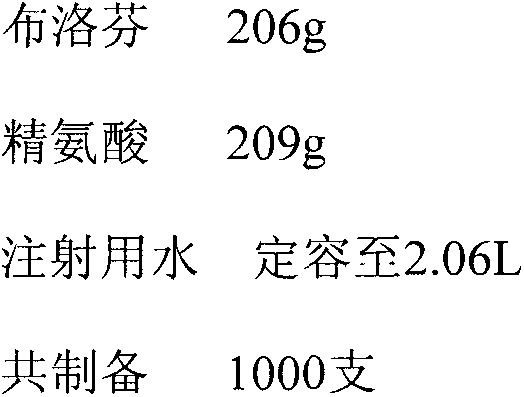
[0050] Preparation of powder injection with arginine: ibuprofen molar ratio of 1.2:1
[0051] prescription
[0052]
[0053] Arginine and ibuprofen were both treated with activated carbon for needles according to the powder injection workshop's requirements for material entry and exit, equipment and utensils, and standard operating procedures for use. Add about 1.8 liters of water for injection in the arginine of 209g, stir at room temperature until dissolving, then add the ibuprofen of 206g in the arginine solution of gained, mix at room temperature until dissolving, add water to 2.06 liters in the gained solution, obtain Molar ratio (arginine: ibuprofen) is the 100mg / ml ibuprofen solution of 1: 1.2, then the medicinal liquid filling after the inspection is qualified in the aseptic antibiotic glass bottle, then the glass bottle is placed in lyophilization After pre-freezing in the machine for 4 hours, sublimation at low temperature for 16 hours, and then drying under redu...
Embodiment 2-4
[0055] The same method as in Example 1 was used to prepare arginine: ibuprofen lyophilized powders with molar ratios of 1.1:1, 1.5:1 and 1.8:1 respectively.
Embodiment 5
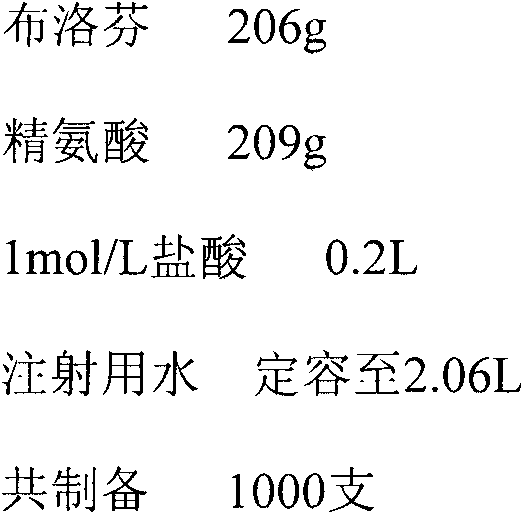
[0057] Preparation of Injection with Ibuprofen Arginine Salt:Arginine Hydrochloride Molar Ratio of 1:0.2
[0058] prescription
[0059]
[0060]The arginine of 209g, the ibuprofen of 206g are added in 1.4 liters of water for injection, after heating and stirring until dissolving, be cooled to 0 ℃, slowly add hydrochloric acid and then be settled to 2.06 liters with water for injection again, obtain molar ratio (arginine Acid: ibuprofen) is 1: 1.2 100mg / ml solution (i.e. ibuprofen arginine salt: arginine hydrochloride molar ratio is 1: 0.2), adds the carbon for needle by 0.15% of total water, Stir at 60°C for 10 to 15 minutes, then filter the resulting solution through a G6 sand rod and a 0.2 μm microporous membrane, and then pack it into a receiving bottle. After passing the clarity inspection, it is filled and sealed. Terminal sterilization, the sterilized product is packaged after vacuum leak detection, uniform dispersion by shaking, cooling and light inspection.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap