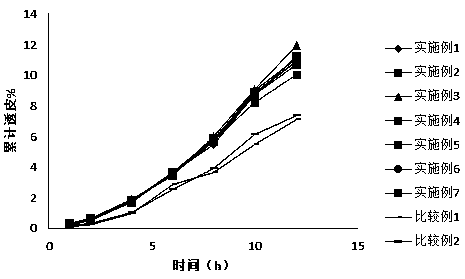
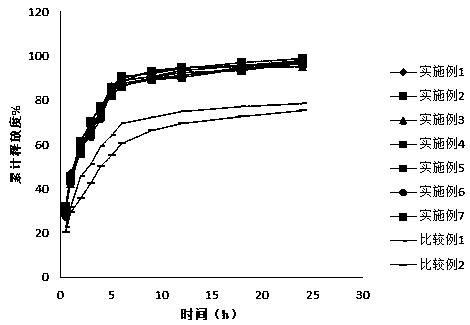
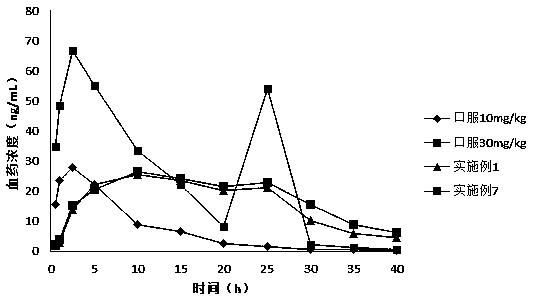
External preparations for skin containing calcium receptor active compounds
A technology for external preparations and calcium receptors, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, and organic active ingredients, etc. Difficulty and other problems, to achieve the effect of drug treatment, reduce the number of doses, and facilitate use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] materials Weight (g) proportion% Cinacalcet hydrochloride 2.5 1.25 Polyacrylic acid 6.0 3.00 Povidone 61.0 30.50 sodium hydroxymethyl cellulose 40.6 20.30 gelatin 19.0 9.50 glycerin 30.0 15.00 Sorbitol 10.5 5.25 Azone 14.0 7.00 Menthol 6.0 3.00 Kaolin 10.0 5.00 Aluminum glycylate 0.4 0.20 purified water Appropriate amount batch 100 stickers
[0028] Preparation process: mix cinacalcet hydrochloride, azone, and menthol in prescribed amounts, stir, and disperse evenly; mix polyacrylic acid, povidone, sodium hydroxymethylcellulose, kaolin, aluminum glycolate, and sorbitol with glycerin Disperse and stir evenly; mix gelatin with an appropriate amount of water to form a gelatin aqueous solution; mix the above three mixed solutions, continue to stir evenly, apply the paste prepared above evenly on the backing layer, cover the protective layer, and cut to Specify the...
Embodiment 2
[0030] materials Weight (g) proportion% Cinacalcet hydrochloride 5.0 2.50 Polyacrylic acid 20.0 10.00 Polyvinylpyrrolidone 38.5 19.25 Povidone 22.5 11.25 gelatin 28.0 14.00 glycerin 71.0 35.50 Azone 8.0 4.00 Oleic acid 4.0 2.00 kaolin 2.8 1.40 Aluminum glycylate 0.2 0.10 purified water Appropriate amount batch 100 stickers
[0031] Preparation process: Mix the prescribed amount of cinacalcet hydrochloride, azone, and oleic acid evenly to obtain the main drug mixture; disperse polyvinylpyrrolidone, povidone, kaolin, and glycol with glycerin, and stir evenly; gelatin Mix evenly with water to obtain gelatin aqueous solution; mix the above three mixed solutions, continue to stir evenly, apply the paste prepared above evenly on the backing layer, cover the protective layer, and cut to the specified size as required.
Embodiment 3
[0033] materials Weight (g) proportion% Cinacalcet hydrochloride 7.5 3.75 carbomer 14.0 7.00 polyethylene glycol 20.0 10.00 gelatin 20.0 10.00 sodium alginate 20.0 10.00 glycerin 100.1 50.05 N-Methylpyrrolidone 10.0 5.00 kaolin 7.8 3.90 Aluminum Glycinate 0.6 0.30 purified water Appropriate amount batch 100 stickers
[0034] Preparation process: Mix the prescribed amount of cinacalcet hydrochloride and N-methylpyrrolidone to obtain the main drug solution; disperse carbomer, polyethylene glycol, sodium alginate, kaolin, and aluminum glycinate evenly with glycerin; gelatin Mix evenly with water to get gelatin aqueous solution; mix the above three mixed solutions, stir evenly, apply the paste prepared above evenly on the backing layer, cover the protective layer, and cut to the specified size as required.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com