Cured aspirin phospholipid composition and preparation method and medicinal preparation thereof
A technology of aspirin phospholipid and aspirin, which can be applied to drug combinations, antipyretics, anti-inflammatory agents, etc., can solve the problems of salicylic acid poisoning, inconvenient medication, gastric mucosal damage, etc., achieves less environmental pollution, and improves medication compliance. Sexual, release-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
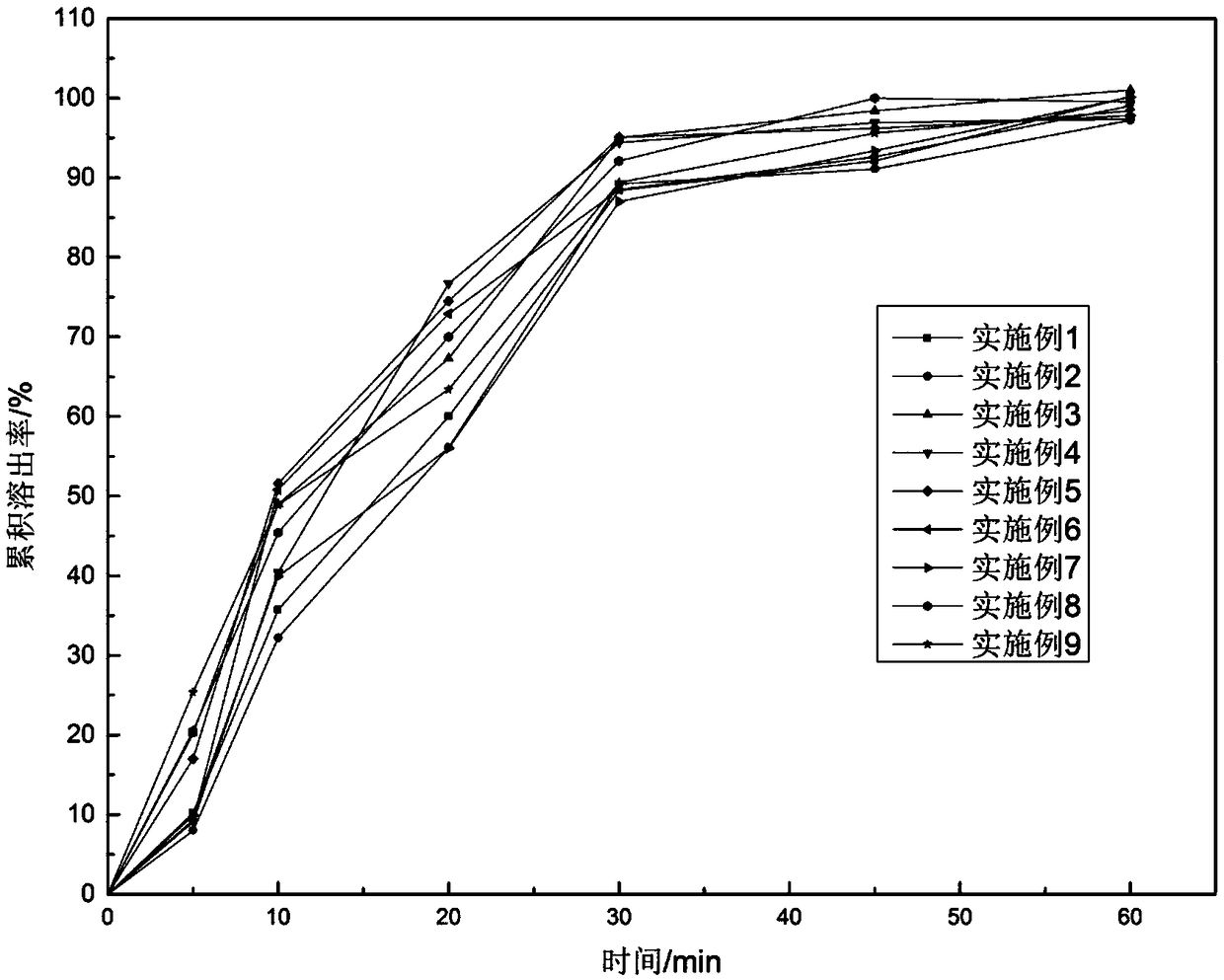
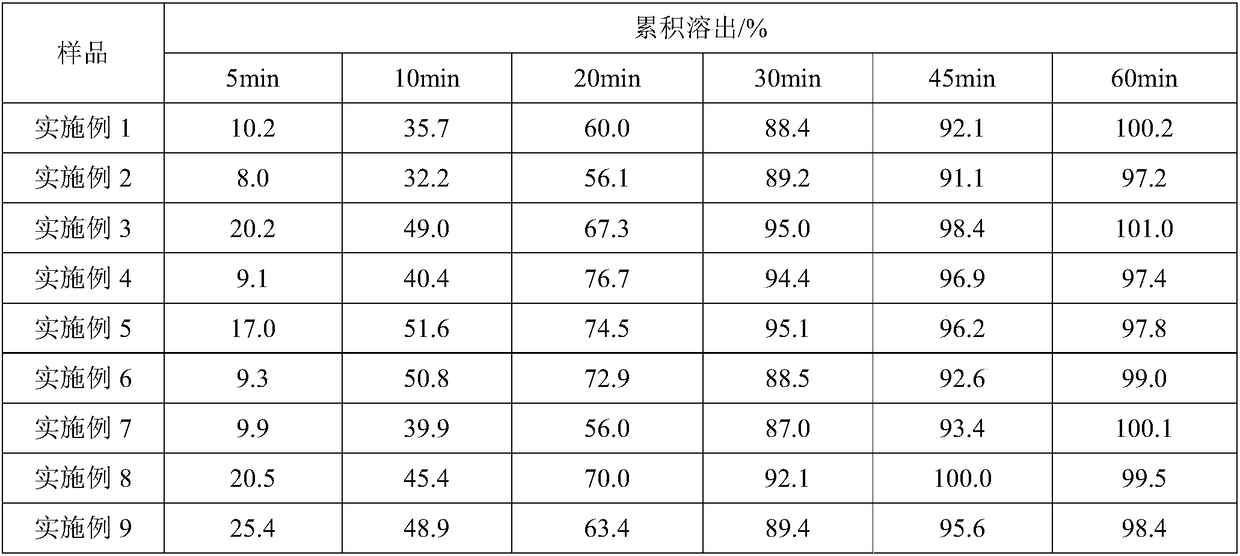
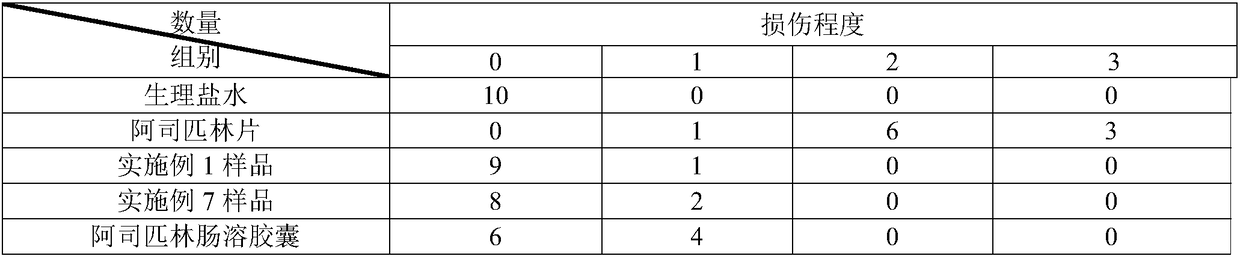
Image
Examples
Embodiment 1
[0038] A kind of aspirin phospholipid solidification composition, it is made by aspirin, liquid phospholipid, adsorbent, viscosity modifier and hydrolysis inhibitor, wherein aspirin 810g, liquid phospholipid 810g, adsorbent 940g, viscosity regulator 179.9g, hydrolysis inhibitor 74.8 g;
[0039] The liquid phospholipid is phosal 35SB, the content of phosphatidylcholine in the liquid phospholipid is 50wt%, the adsorbent is silicon dioxide, the viscosity regulator is glycerol tricaprylate, and the hydrolysis inhibitor is citric acid.
[0040] A preparation method of aspirin phospholipid solidified composition, prepared by the following steps:
[0041] a) Crush aspirin to its D 90 20μm, spare;
[0042] b) Put the liquid phospholipid in a container, heat and stir in a water bath, and vacuumize and degas at the same time; after the degassing is completed, add a hydrolysis inhibitor, a viscosity modifier and aspirin in a water bath and vacuumize the environment, and then stir evenl...
Embodiment 2
[0046] A kind of aspirin phospholipid solidification composition, it is made by aspirin, liquid phospholipid, adsorbent, viscosity modifier and hydrolysis inhibitor, wherein aspirin 270g, liquid phospholipid 810g, adsorbent 540g, viscosity regulator 79.9g, hydrolysis inhibitor 44.8 g; the liquid phospholipid is phosal 35SB, the content of phosphatidylcholine in the liquid phospholipid is 50wt%, the adsorbent is made of amino-modified mesoporous silica, the viscosity modifier is glycerol tricaprylate, and the hydrolysis inhibitor is citron acid;
[0047] Amino-modified mesoporous silica is prepared by the following method, prepare 80 parts by weight of ammonium nitrate aqueous solution with a pH value of 6.0, dissolve 0.6 parts by weight of cetyltrimethylammonium bromide in aqueous ammonium nitrate solution, dissolve and stir After defoaming naturally, then the above solution is preheated to 80°C, followed by adding 4 parts by weight of tetraethyl orthosilicate and stirring at ...
Embodiment 3
[0054] A kind of aspirin phospholipid solidification composition, it is made by aspirin, liquid phospholipid, adsorbent, viscosity modifier and hydrolysis inhibitor, wherein aspirin 810g, liquid phospholipid 810g, adsorbent 940g, viscosity regulator 259.8g, hydrolysis inhibitor 74.8 g;
[0055] The liquid phospholipid is a mixture of phosal 35SB and phosal 53MCT in a weight ratio of 1:1, the content of phosphatidylcholine in the liquid phospholipid is 50wt%, the adsorbent is silicon dioxide, the viscosity regulator is glycerol tricaprylate, hydrolyzed The inhibitor is citric acid.
[0056] A preparation method of aspirin phospholipid solidified composition, prepared by the following steps:
[0057] a) Crush aspirin to its D 90 20μm, spare;
[0058] b) Put the liquid phospholipid in a container, heat and stir in a water bath, and vacuumize and degas at the same time; after the degassing is completed, add a hydrolysis inhibitor, a viscosity modifier and aspirin in a water bat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com