Medicine composite containing aspirin and application thereof
An aspirin and composition technology, which is applied in the field of pharmaceutical compositions containing low-dose aspirin, can solve the problems of not preventing or treating hypertension complications and the like, and achieves good sympathomimetic activity, good antihypertensive effect, and prevents smooth muscle cell proliferation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
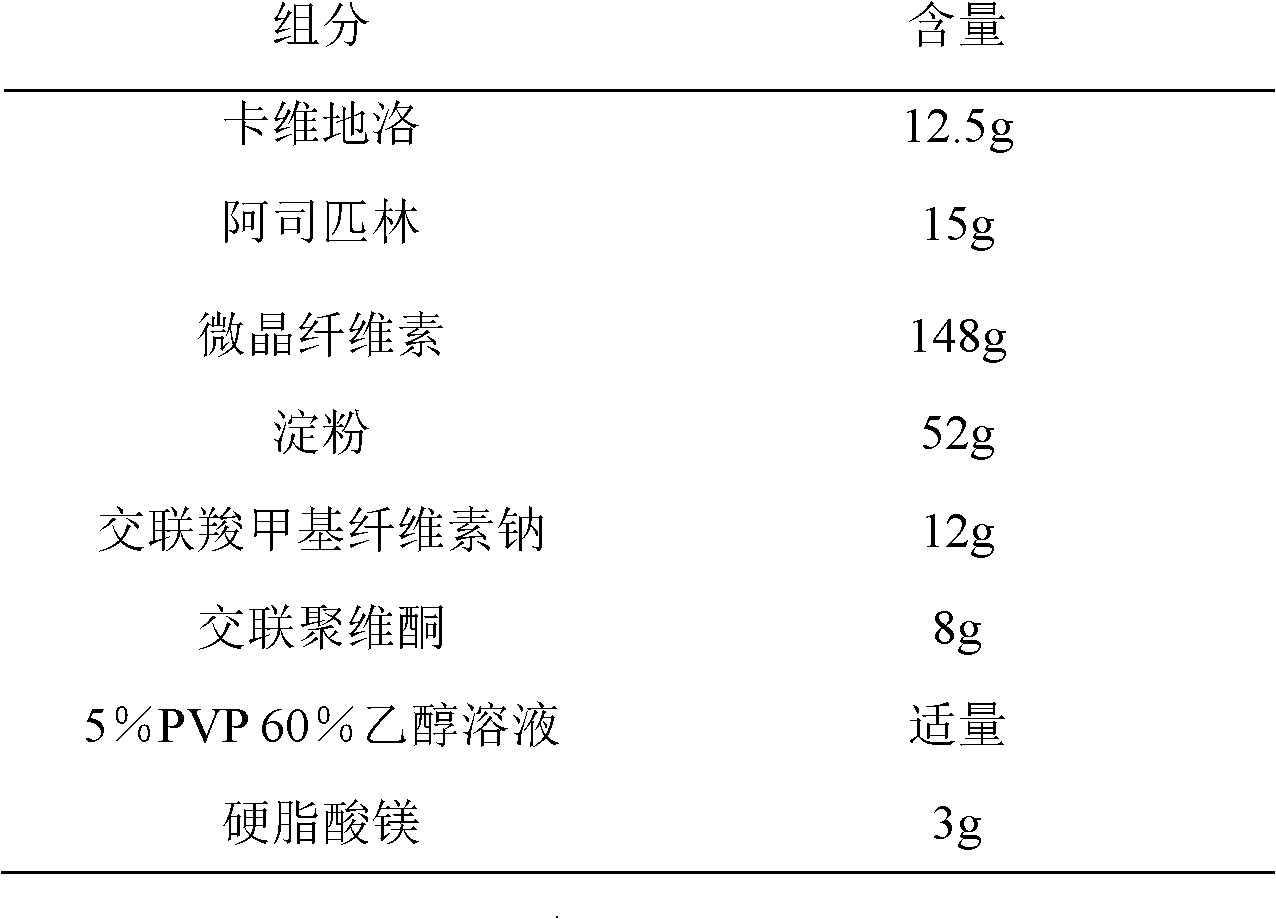
[0021] The preparation of embodiment 1 carvedilol / aspirin compound tablet
[0022] Prescription composition:
[0023]
[0024]
[0025] Preparation Process:
[0026] Take carvedilol and aspirin according to the prescription quantity, take microcrystalline cellulose and starch as fillers, croscarmellose sodium and crospovidone as disintegrants, and 5% PVP 60% ethanol solution is Adhesive, magnesium stearate as lubricant, one-step granulation with fluidized bed, and then compressed into tablets, to obtain.
Embodiment 2
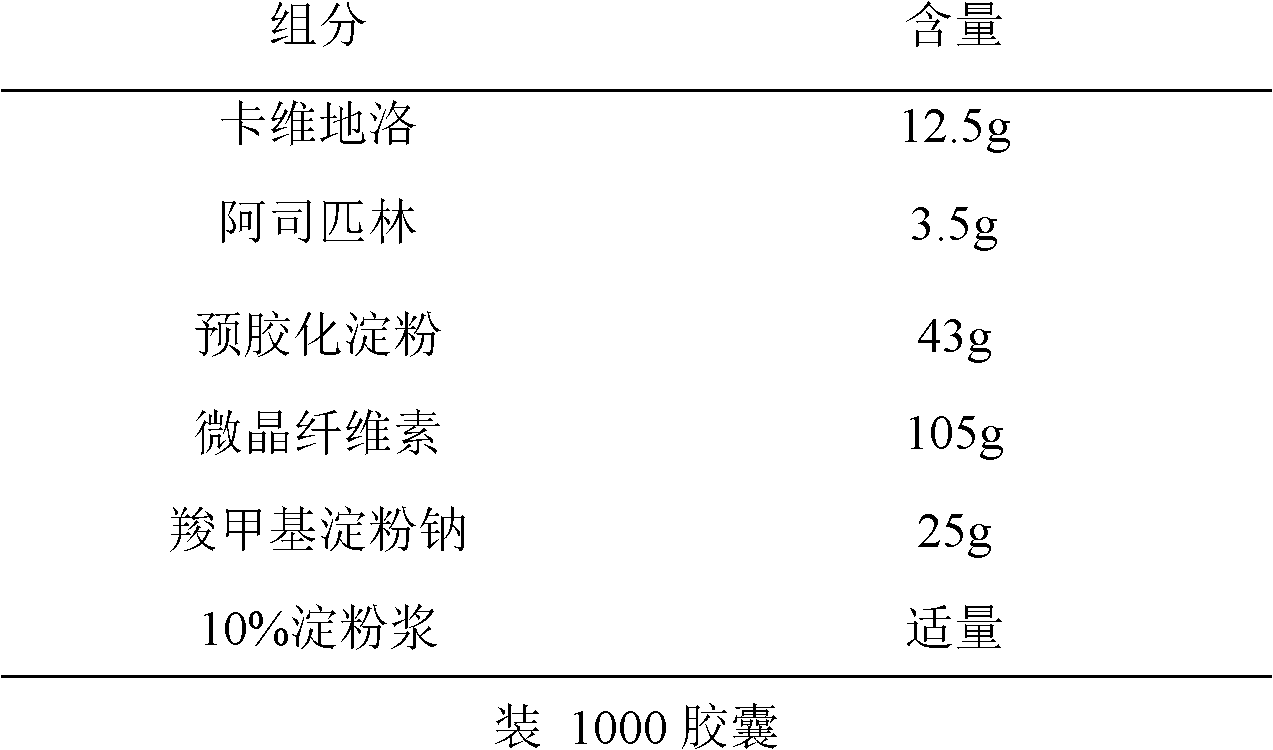
[0027] The preparation of embodiment 2 carvedilol / aspirin compound capsules
[0028] Prescription composition:
[0029]
[0030] Preparation process: pass the carvedilol, aspirin, pregelatinized starch, microcrystalline cellulose and sodium carboxymethyl starch in the prescription through a 100-mesh sieve, weigh according to the prescription amount, mix well, add 10% starch slurry in an appropriate amount Granulate, dry below 55°C, sieve through a 18-mesh sieve, and fill the capsules.
Embodiment 3
[0031] Example 3 Curative Effect Study of Carvedilol Combined with Aspirin on Hypertensive Rats
[0032] Spontaneous hereditary hypertensive rats (SHR) were purchased from Shanghai Slack Experimental Animal Co., Ltd. Grouping of animals: 60 SHR rats of 200-250 g were randomly divided into 4 groups according to blood pressure level, 15 rats in each group. Followed by model control group, carvedilol group, aspirin group and combined drug group. Another 10 SD rats were used as normal blood pressure control group. Rats in each group were intragastrically administered once a day in the morning and evening, and given the corresponding test substances according to the dosage in Table 1.
[0033] Table 1. Grouping and administration of experimental animals
[0034]
[0035]
[0036] After 15 weeks of intragastric administration to the rats in each group, the urine of the rats was inoculated with metabolic cages, and the microalbumin content in the urine of the rats in each gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com