Method for preparing aspirin enteric-coated tablet for removing fever, easing pain and resisting inflammation
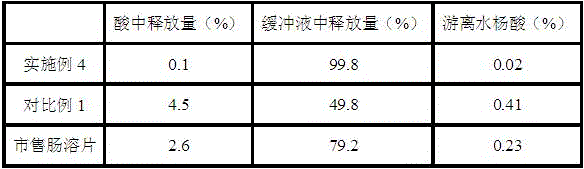
一种阿司匹林、抗炎药物的技术,应用在医药领域,能够解决用药不方便、水杨酸中毒反应、胃黏膜损伤等问题,达到降低胃肠道不良反应、低游离水杨酸含量的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
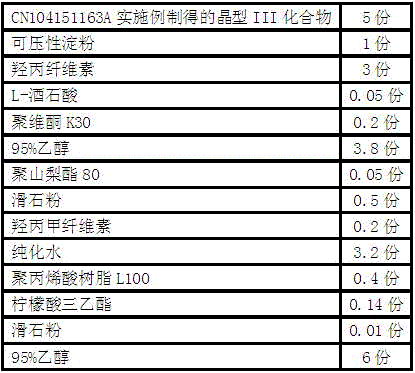
Embodiment 1
[0046] The preparation of embodiment 1 aspirin compound
[0047] (1) Add the aspirin crude product to a mixed solution of absolute ethanol, ethyl acetate, and cyclohexane whose volume is 5 times the weight of aspirin, and the volume ratio of absolute ethanol, ethyl acetate, and cyclohexane is: 4.5: 1.5:1, heat up to 35°C, stir until completely dissolved;
[0048] (2) In a sound field with a frequency of 25KHz and an output power of 40W, add a mixed solution of ether and water whose volume is 10 times the weight of aspirin while stirring. The volume ratio of ether and water is 1:3.5, and the stirring speed is 150 rpm / min, adding speed is 80ml / min;
[0049] (3) After adding the mixed solution of ether and water, under a sound field with a frequency of 15KHz and an output power of 10W, cool down to 0-2°C at a rate of 2°C / hour, grow crystals for 3 hours, wash, and dry in vacuum to obtain aspirin compound.
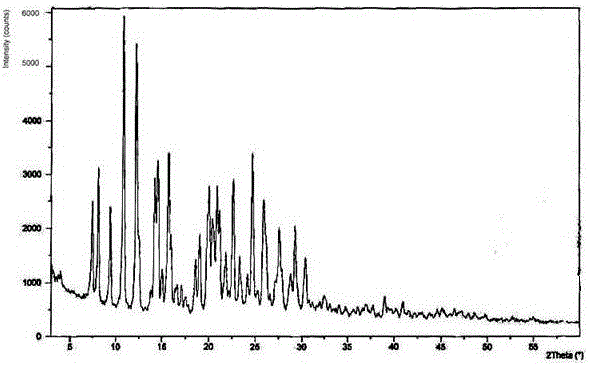
[0050] The obtained aspirin compound was measured by powder X-ray powd...
Embodiment 2
[0051] The preparation of embodiment 2 aspirin compounds
[0052] (1) Add the aspirin crude product to a mixed solution of absolute ethanol, ethyl acetate, and cyclohexane whose volume is 6 times the weight of aspirin, and the volume ratio of absolute ethanol, ethyl acetate, and cyclohexane is: 4.5: 1.5:1, heat up to 40°C, stir until completely dissolved;
[0053] (2) In a sound field with a frequency of 27.5KHz and an output power of 50W, add a mixed solution of ether and water whose volume is 12.5 times the weight of aspirin while stirring. The volume ratio of ether and water is 1:3.5, and the stirring speed is 205 rev / min, adding speed is 100 ml / min;
[0054] (3) After adding the mixed solution of ether and water, under a sound field with a frequency of 17.5KHz and an output power of 15W, the temperature was lowered to 0-2°C at a rate of 3°C / hour, the crystal was grown for 4.5 hours, washed, and dried in vacuum to obtain Aspirin compound.
[0055] The obtained aspirin co...
Embodiment 3
[0056] The preparation of embodiment 3 aspirin compounds
[0057] (1) Add the aspirin crude product to a mixed solution of absolute ethanol, ethyl acetate, and cyclohexane whose volume is 7 times the weight of aspirin, and the volume ratio of absolute ethanol, ethyl acetate, and cyclohexane is: 4.5: 1.5:1, heat up to 45°C, stir until completely dissolved;
[0058] (2) In a sound field with a frequency of 30KHz and an output power of 60W, add a mixed solution of ether and water whose volume is 15 times the weight of aspirin while stirring. The volume ratio of ether and water is 1:3.5, and the stirring speed is 260 rpm / min, adding speed is 120ml / min;
[0059] (3) After adding the mixed solution of ether and water, under a sound field with a frequency of 20KHz and an output power of 20W, the temperature is lowered to 0-2°C at 4°C / hour, the crystal is grown for 6 hours, washed, and dried in vacuum to obtain aspirin compound.
[0060] The obtained aspirin compound was measured ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com