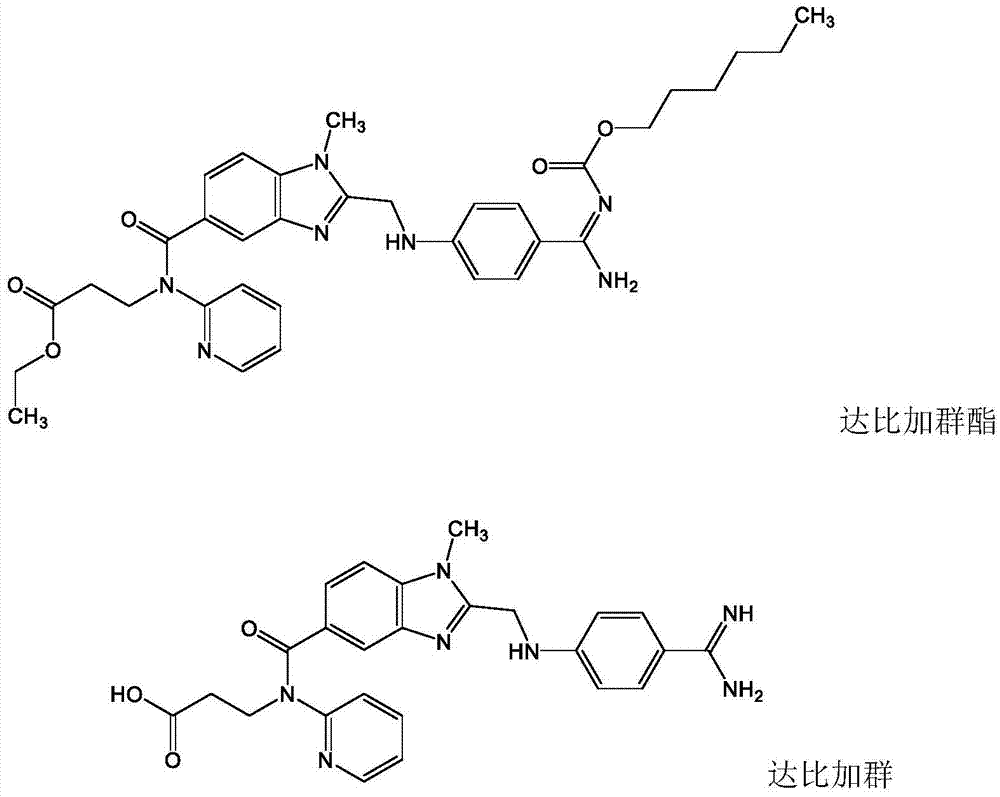
High-bioavailability dabigatran etexilate pharmaceutical composition
A technology for dabigatran etexilate and dabigatran etexilate mesylate, which is applied in the field of high bioavailability dabigatran etexilate pharmaceutical compositions, can solve problems such as gastrointestinal adverse reactions, and achieve gastric protection. Intestinal mucosa, reducing the risk of recurrence, and improving the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
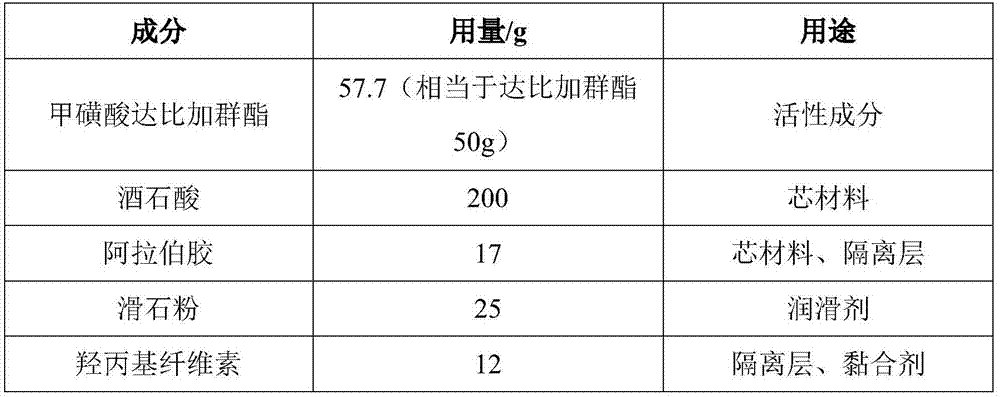
[0028] Embodiment 1 dabigatran etexilate mesylate, betaine hydrochloride pellet capsule
[0029]
[0030] According to the prescription in the above table, adopt the method of patent CN101632668A embodiment 1 to prepare dabigatran etexilate mesylate betaine hydrochloride pellets (replace tartaric acid in the method with betaine hydrochloride).
[0031] The micropills can be packed into capsules or directly packed, and the clinical dosage is 75 mg to 300 mg based on dabigatran etexilate, so as to adjust different loading amounts. For example, each dosage can be 75 mg of dabigatran etexilate (the corresponding amount of betaine hydrochloride is 750 mg), and 150 mg of dabigatran etexilate (the corresponding amount of betaine hydrochloride is 1500 mg).
Embodiment 2
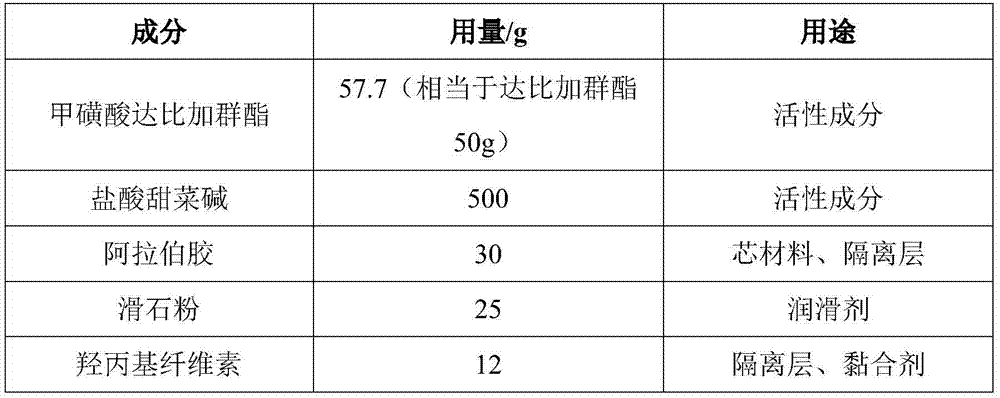
[0060] Embodiment 2 dabigatran etexilate mesylate, betaine citrate pellet capsule
[0061] Element Dosage / g use Dabigatran etexilate mesylate 57.7 (equivalent to dabigatran etexilate 50g) active ingredient betaine citrate 200 active ingredient microcrystalline cellulose 100 filler Micropowder silica gel 10 lubricant Hydroxypropyl Cellulose 35 Adhesive
[0062] 1. Preparation of betaine citrate pellet core
[0063] Take the prescription amount of betaine citrate, pulverize and sieve; take microcrystalline cellulose and pass it through an 80-mesh sieve; mix betaine hydrochloride and microcrystalline cellulose evenly, add water to prepare soft material, and make pellets by extrusion spheronization, and dry.
[0064] 2. Pack isolation gown
[0065] The betaine hydrochloride pellets prepared in step 1 were placed in a fluidized bed, and a 3% HMPC aqueous solution was used as a coating solution for bottom spray coating, and...
Embodiment 3
[0070] Embodiment 3 dabigatran etexilate mesylate, betaine hydrochloride compression-coated tablet
[0071] Prescription (1000 tablets):
[0072] Tablet composition Dosage / g Dabigatran etexilate mesylate 86.5 (equivalent to dabigatran etexilate 75g) lactose monohydrate 80 microcrystalline cellulose 55.5 Magnesium stearate 3.5 Polyvinylpyrrolidone 4.5
[0073] Preparation Process:
[0074] (1) Mix dabigatran etexilate mesylate, lactose monohydrate and microcrystalline cellulose evenly.
[0075] (2) Make polyvinylpyrrolidone into 10% ethanol solution of polyvinylpyrrolidone as an adhesive.
[0076] (3) Add the 10% polyvinylpyrrolidone ethanol solution prepared in the step (2) to the step (1) to make a soft material.
[0077] (4) Pass the soft material prepared in step (3) through a 20-mesh sieve for wet granulation.
[0078] (5) Dry the granules prepared in step (4) at 60° C. for 0.5 h.
[0079] (6) Pass the granules in step (5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com