A kind of synthetic method of cefditoren pivoxil mother nucleus
A technology of cefditoren pivoxil and a synthesis method, which is applied in directions such as organic chemistry to achieve the effects of easy operation, high product purity and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
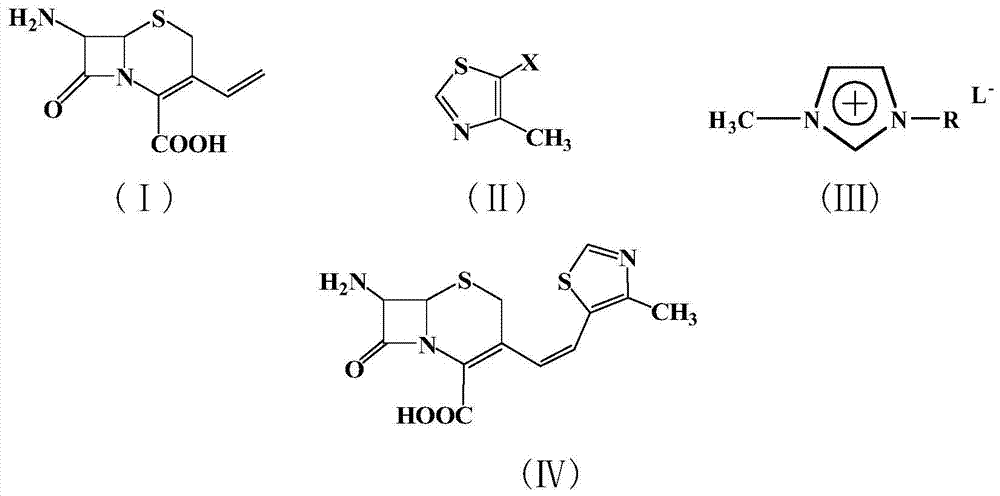
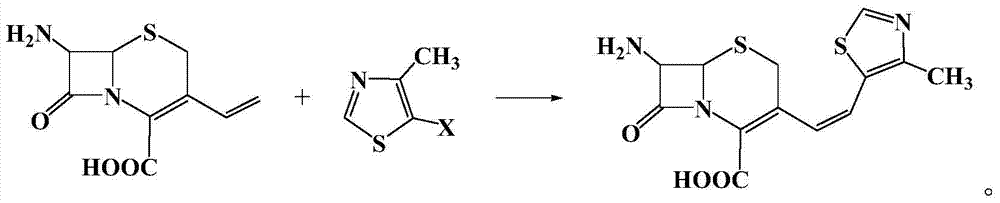
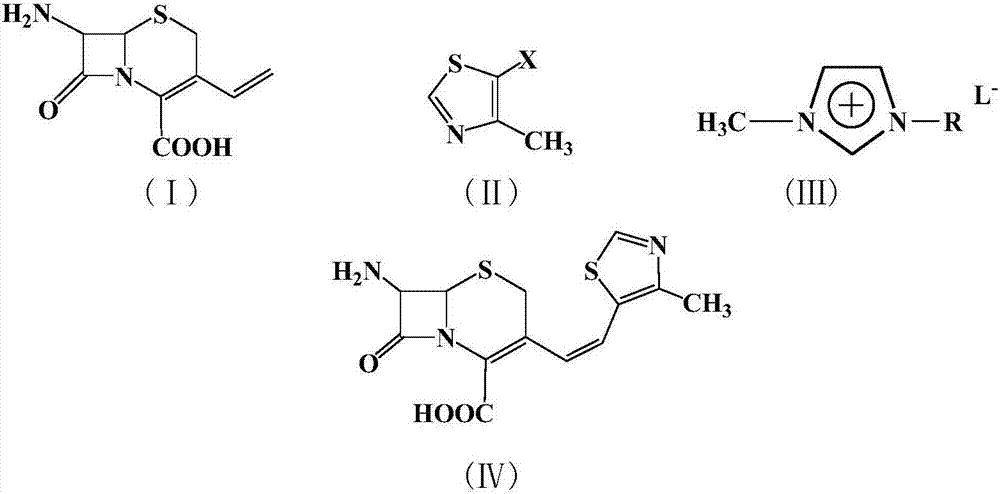
[0020] Example 1: In a 100 ml three-necked flask, add 2.26 g (10 mmol) of 7-amino-3-vinyl-3-cephem-4-carboxylic acid, 1.34 g (10 mmol) of 4-methyl-5-chlorothiazole , 22.6g of 1-methyl-3-butylimidazolium tetrafluoroborate ionic liquid, 0.224g (1mmol) of palladium acetate, 0.26g (1mmol) of triphenylphosphine, and 1.0g (10mmol) of triethylamine were added to the reactor , react at 50°C for 10 hours, after the reaction is complete, add saturated sodium bicarbonate solution to adjust the pH to 8-10, extract the reaction solution with 5×3 mL of dichloromethane for 3 times, dry the extract with anhydrous sodium sulfate, and concentrate , recrystallized from ethanol to obtain 2.62 g of a light yellow solid product with a yield of 81%. 99% pure. Melting point: Decompose at 220°C. MS: m / e=323 (M + ).
Embodiment 2
[0021] Example 2: In a 100 ml three-necked flask, add 7-amino-3-vinyl-3-cephem-4-carboxylic acid 2.26g (10mmol), 4-methyl-5-bromothiazole 1.78g (10mmol) , 22.6g of 1-methyl-3-butylimidazolium tetrafluoroborate ionic liquid, 0.224g (1mmol) of palladium acetate, 0.26g (1mmol) of triphenylphosphine, and 1.0g (10mmol) of triethylamine were added to the reactor , react at 50°C for 5 hours, after the reaction is completed, add saturated sodium bicarbonate solution to adjust the pH to 8-10, extract the reaction solution with 5×3 mL of dichloromethane for 3 times, dry the extract with anhydrous sodium sulfate, and concentrate , recrystallized from ethanol to obtain 2.72 g of a light yellow solid product, with a yield of 84%. 99% pure. Melting point: Decompose at 220°C. MS: m / e=323 (M + ).
Embodiment 3
[0022] Example 3: In a 100 ml three-necked flask, add 2.26 g (10 mmol) of 7-amino-3-vinyl-3-cephem-4-carboxylic acid, 2.25 g (10 mmol) of 4-methyl-5-iodothiazole , 22.6g of 1-methyl-3-butylimidazolium tetrafluoroborate ionic liquid, 0.224g (1mmol) of palladium acetate, 0.26g (1mmol) of triphenylphosphine, and 1.0g (10mmol) of triethylamine were added to the reactor , react at 10°C for 10 hours, after the reaction is completed, add saturated sodium bicarbonate solution to adjust the pH to 8-10, extract the reaction solution with 5×3 mL of dichloromethane for 3 times, dry the extract with anhydrous sodium sulfate, and concentrate , recrystallized from ethanol to obtain 2.65 g of a light yellow solid product, with a yield of 82%. 99% pure. Melting point: Decompose at 220°C. MS: m / e=323 (M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com