Antibodies recognizing alpha-synuclein
A technology of synuclein and antibody, applied in the direction of antibody, immunoglobulin, antibody medical components, etc., can solve problems such as poisoning nerves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0237] Example 1: Isolation of mouse 5C1
[0238] The mouse 5C1 antibody was produced in mice injected with a polypeptide conjugate containing the polypeptide immunogen VDPDNEAYEGGC (SEQ ID NO: 14) coupled with a goat anti-mouse antibody. The polypeptide contains residues 118 to 126 of α-synuclein fused to the C-terminal end of the GGC polypeptide, and is coupled to the goat antibody through a maleimide linker that binds to the C-terminal cysteine residue. Mouse antibodies.
Embodiment 2
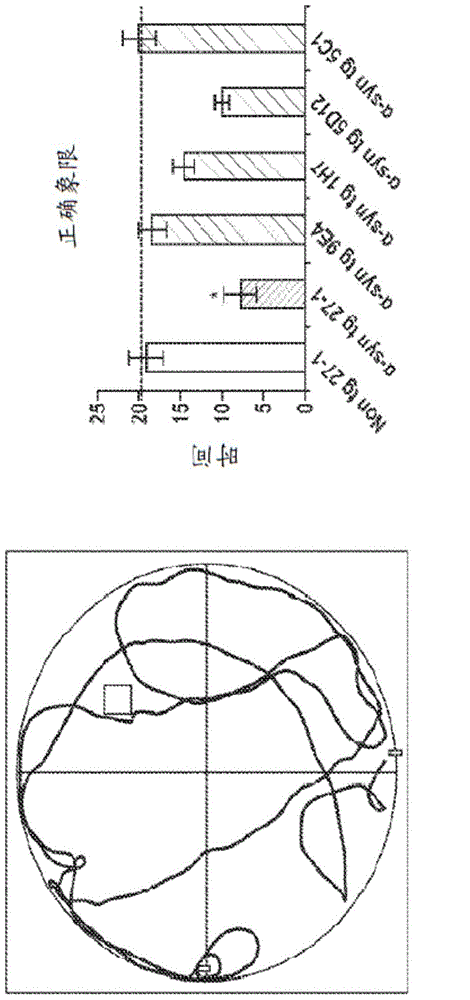
[0239] Example 2: Passive immunization of α-synuclein antibody
[0240] In order to test the effect of α-synuclein antibodies in Lewy body disease animal models, a variety of α-synuclein antibodies were used to passively immunize mice. Wild-type, α-synuclein knock-out and α-synuclein transgenic (line 61, line 61) female mice aged 3 to 4 months were used. The antibodies tested include:
[0241] 9E4 (IgG1, epitope: amino acids 118-126 of α-synuclein);
[0242] 5C1 (IgG1, immunogen: amino acids 118-126 of α-synuclein, cys-linker);
[0243] 5D12 (IgG2, immunogen: amino acids 118-126 of α-synuclein, n-linker);
[0244] 1H7 (IgG1, epitope: amino acids 91-99 of α-synuclein); and
[0245] 27-1 (IgG1 control antibody).
[0246] The mice received a dose of 10 mg / kg of antibody over a period of 5 months, for a total of 21 injections. In addition, the mouse was injected with a lentiviral vector (LV) expressing human α-synuclein (wt), unilaterally introducing human α-synuclein (wt) into the hippoca...
Embodiment 3
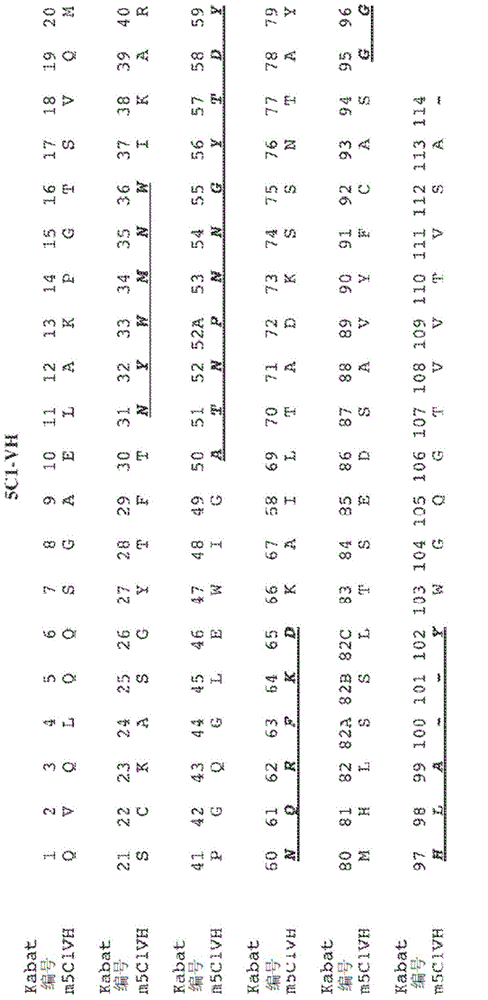
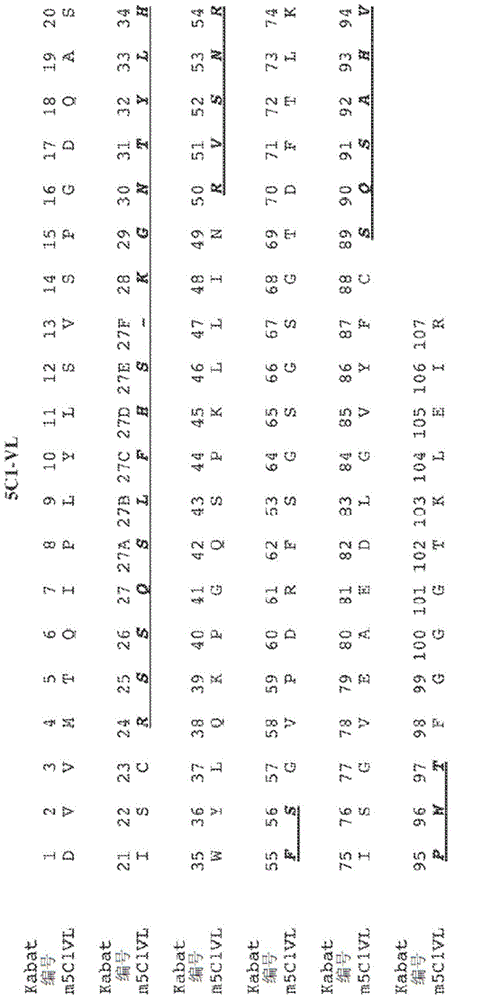
[0252] Example 3: 5C1 variable domain sequencing
[0253] use The mRNA kit is used to extract and purify mRNA from 5C1 hybridoma cells. Then use oligo dT antisense primers and The II kit transcribes purified mRNA into cDNA. By PCR, degenerate VH and VL forward primers and gene-specific (CH / CL) antisense primers were used to amplify the nucleic acid sequences encoding the 5C1 heavy chain and light chain variable regions from cDNA. PCR products designed to include sequences of signal peptide, variable domain and constant region (depending on the antisense primer) are gel purified, cloned into blunt vector or TA vector, and then sequenced. The sequence was deduced from the analysis of at least 3 independent clones with an open reading frame starting from methionine and extending from the variable region to the constant region.
[0254] The nucleic acid sequence encoding the 5C1 heavy chain variable region is shown in SEQ ID NO: 5, and its corresponding protein sequence ( figure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com