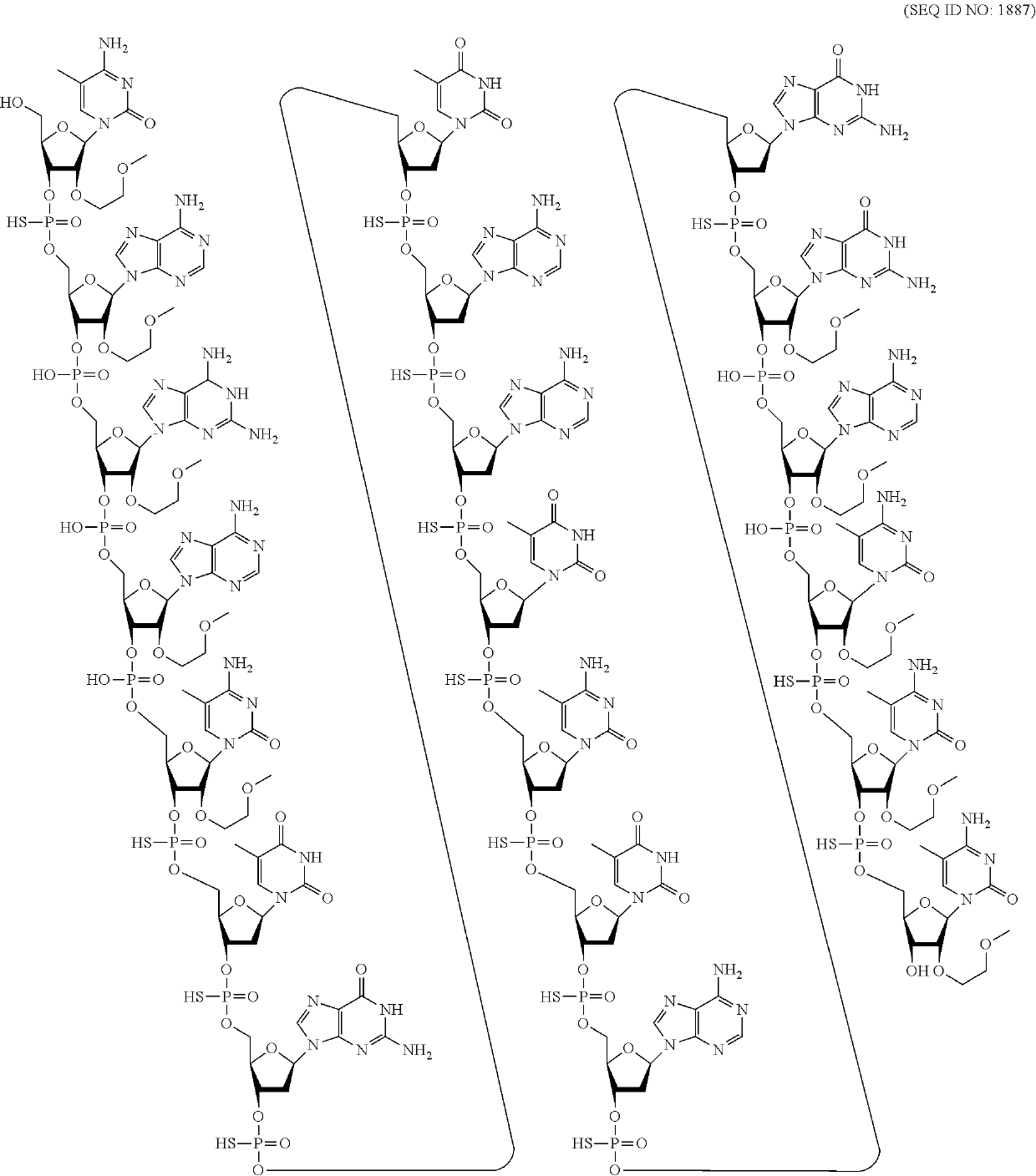
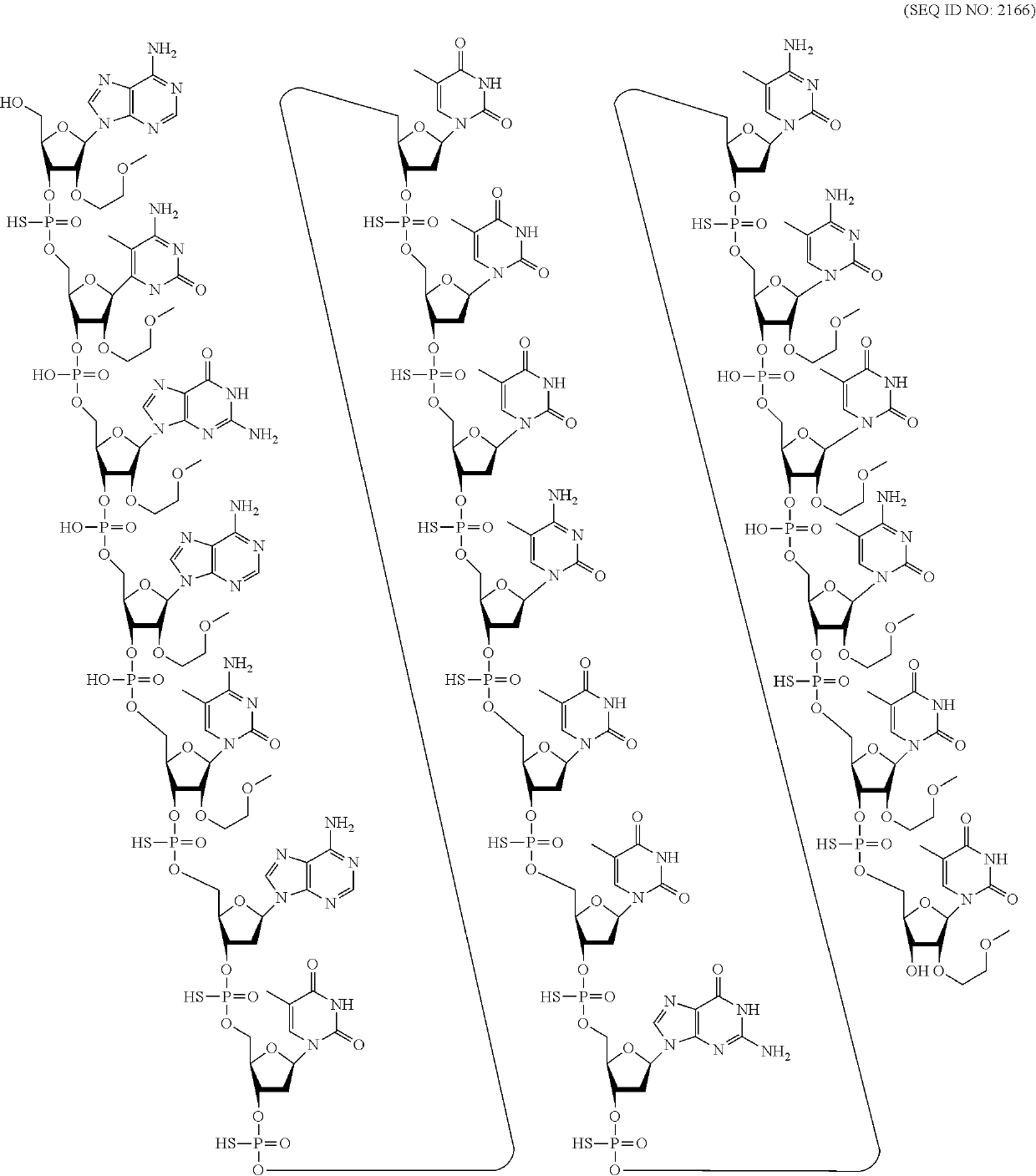
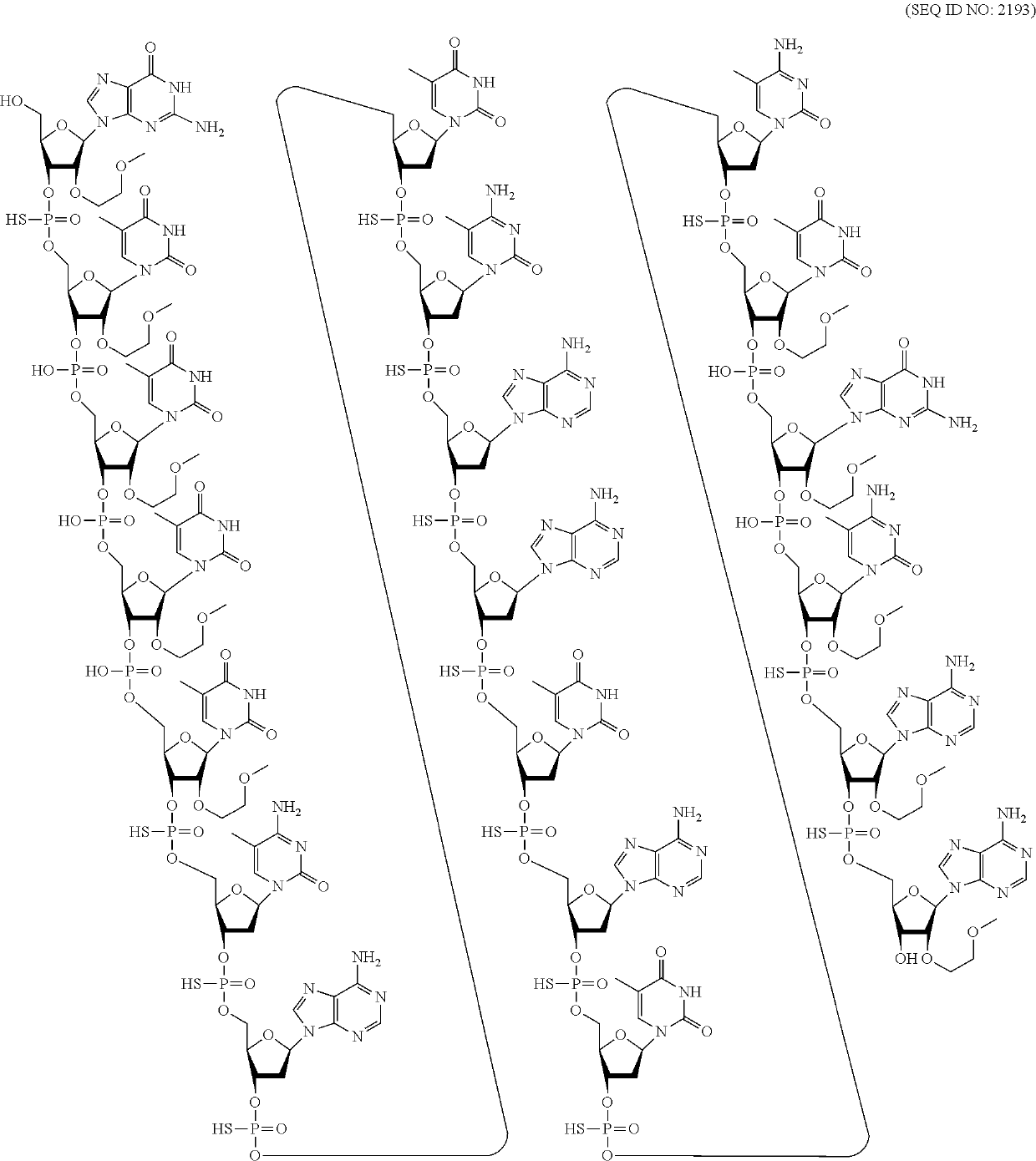
Compounds and methods for reducing snca expression
a technology of compound and method, applied in the field of compound and method for reducing snca expression, can solve the problems of lack of acceptable options for treating neurodegenerative diseases, and achieve the effects of reducing neurodegeneration, improving motor function, and reducing alpha-synuclein aggregates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5-8-4 MOE and cEt Gapmers with Mixed Internucleoside Linkages on Human SNCA In Vitro, Single Dose
[0381]Modified oligonucleotides complementary to a human SNCA nucleic acid were designed and tested for their effect on SNCA mRNA in vitro. The modified oligonucleotides were tested in a series of experiments that had similar culture conditions.
[0382]Cultured SH-SY5Y cells at a density of 20,000 cells per well were transfected using electroporation with 7,000 nM concentration of modified oligonucleotide or no modified oligonucleotide for untreated controls. After approximately 24 hours, RNA was isolated from the cells and SNCA mRNA levels were measured by quantitative real-time PCR Human primer probe set RTS2621 (forward sequence ACGAACCTGAAGCCTAAGAAATATCT, designated herein as SEQ ID NO: 11; reverse sequence GAGCACTTGTACAGGATGGAACAT, designated herein as SEQ ID NO: 12; probe sequence TGCTCCCAAGTTTCTTGAGATCTGCTGACA, designated herein as SEQ ID: 13) was used to measure mRNA levels. SNCA m...
example 2
4-9-4 MOE and cEt Gapmers with Mixed Internucleoside Linkages on Human SNCA In Vitro, Single Dose
[0385]Modified oligonucleotides complementary to a human SNCA nucleic acid were designed and tested as described in Example 1 for their effect on SNCA mRNA in vitro. The modified oligonucleotides were tested in a series of experiments that had similar culture conditions.
[0386]The modified oligonucleotides marked with an asterisk (*) target the amplicon region of the primer probe set. Additional assays may be used to measure the potency and efficacy of oligonucleotides targeting the amplicon region. Compound No. 387978, previously disclosed in WO 2012 / 068405 was also tested and is a comparator oligonucleotide. Compound No. 387978 is a 5-10-5 MOE gapmer wherein each internucleoside linkage is a phosphorothioate internucleoside linkage and each cytosine residue is a 5-methyl cytosine.
[0387]The modified oligonucleotides in tables 7-13 are 4-9-4 MOE and cEt gapmers. The gapmers are 17 nucleob...
example 3
4-9-4 MOE and cEt Gapmers with Mixed Internucleoside Linkages on Human SNCA In Vitro, Single Dose
[0389]Modified oligonucleotides complementary to a human SNCA nucleic acid were designed and tested as described in Example 1 for their effect on SNCA mRNA in vitro. The modified oligonucleotides were tested in a series of experiments that had similar culture conditions.
[0390]The modified oligonucleotides in tables 14-23 are 4-9-4 MOE and cEt gapmers. The gapmers are 17 nucleobases in length, wherein the central gap segment comprises nine 2′-deoxynucleosides and is flanked by wing segments on both the 5′ end on the 3′ end comprising two 2′-MOE nucleosides and two cEt nucleosides. The sugar motif for the gapmers is (from 5′ to 3′): eekkdddddddddkkee; wherein ‘d’ represents a 2′-deoxyribose sugar; ‘e’ represents a 2′-MOE modified sugar; and ‘k’ represents a cEt modified sugar. All cytosine residues throughout each gapmer are 5-methyl cytosines. The internucleoside linkages are mixed phosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com