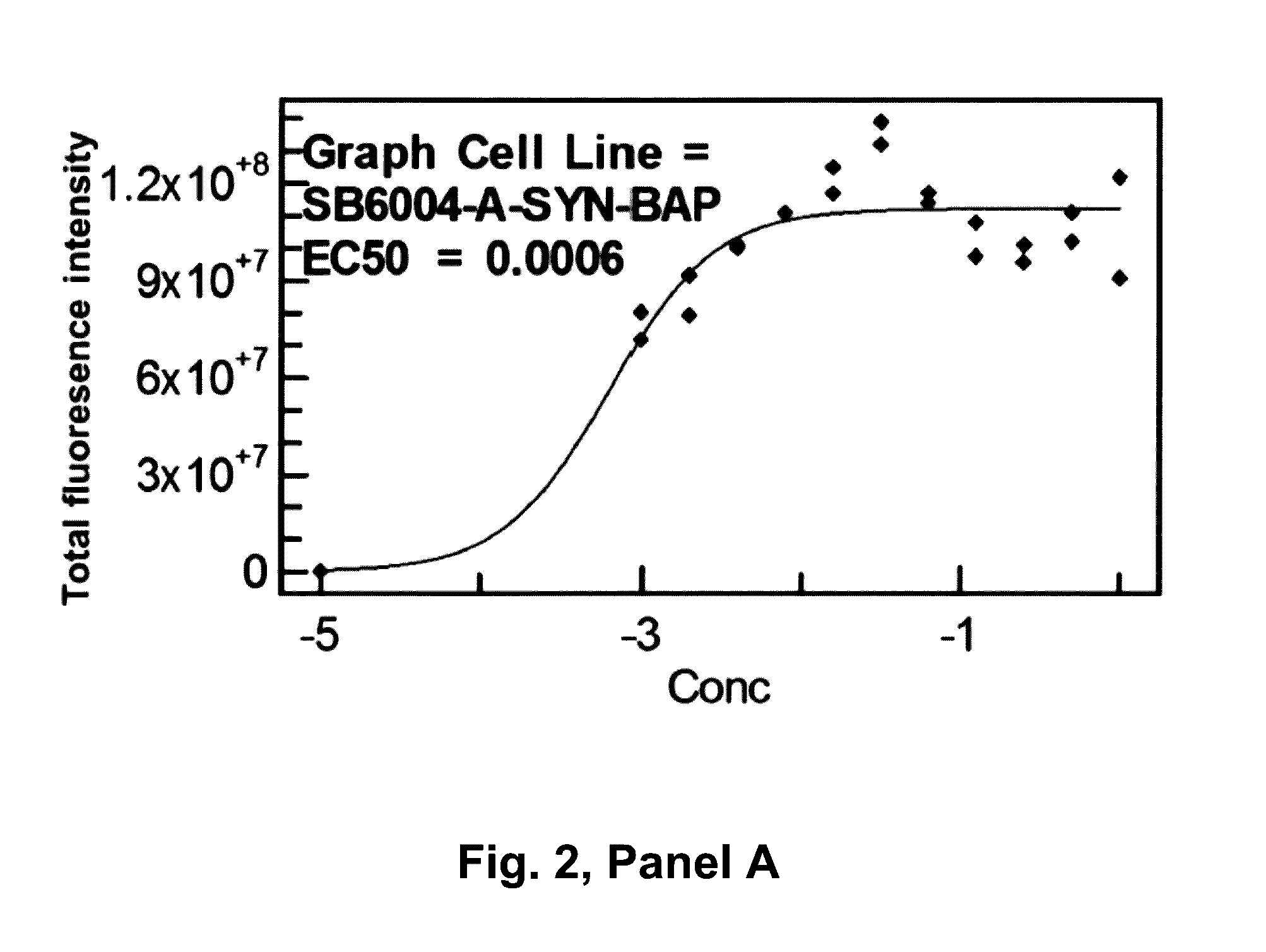
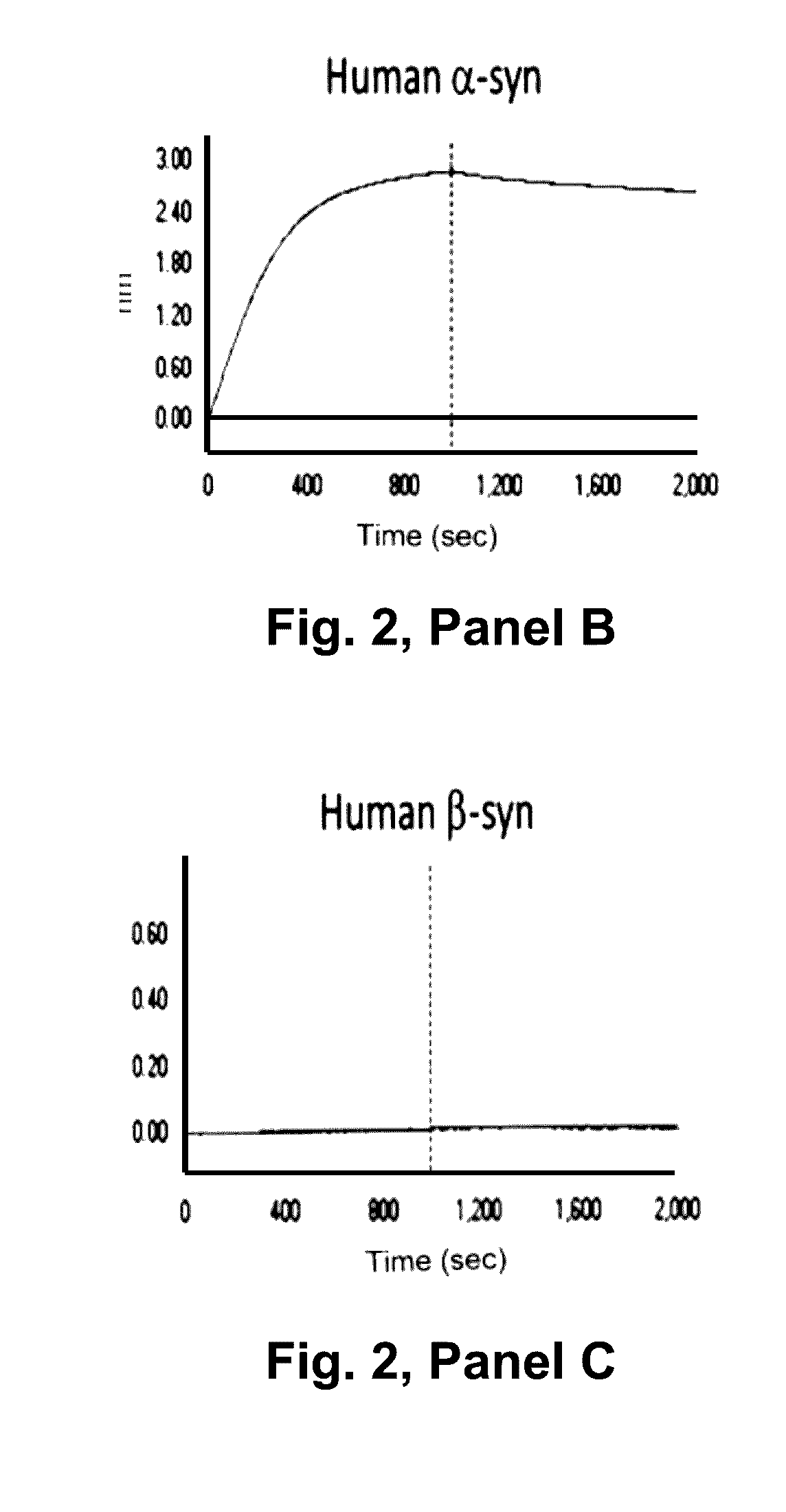
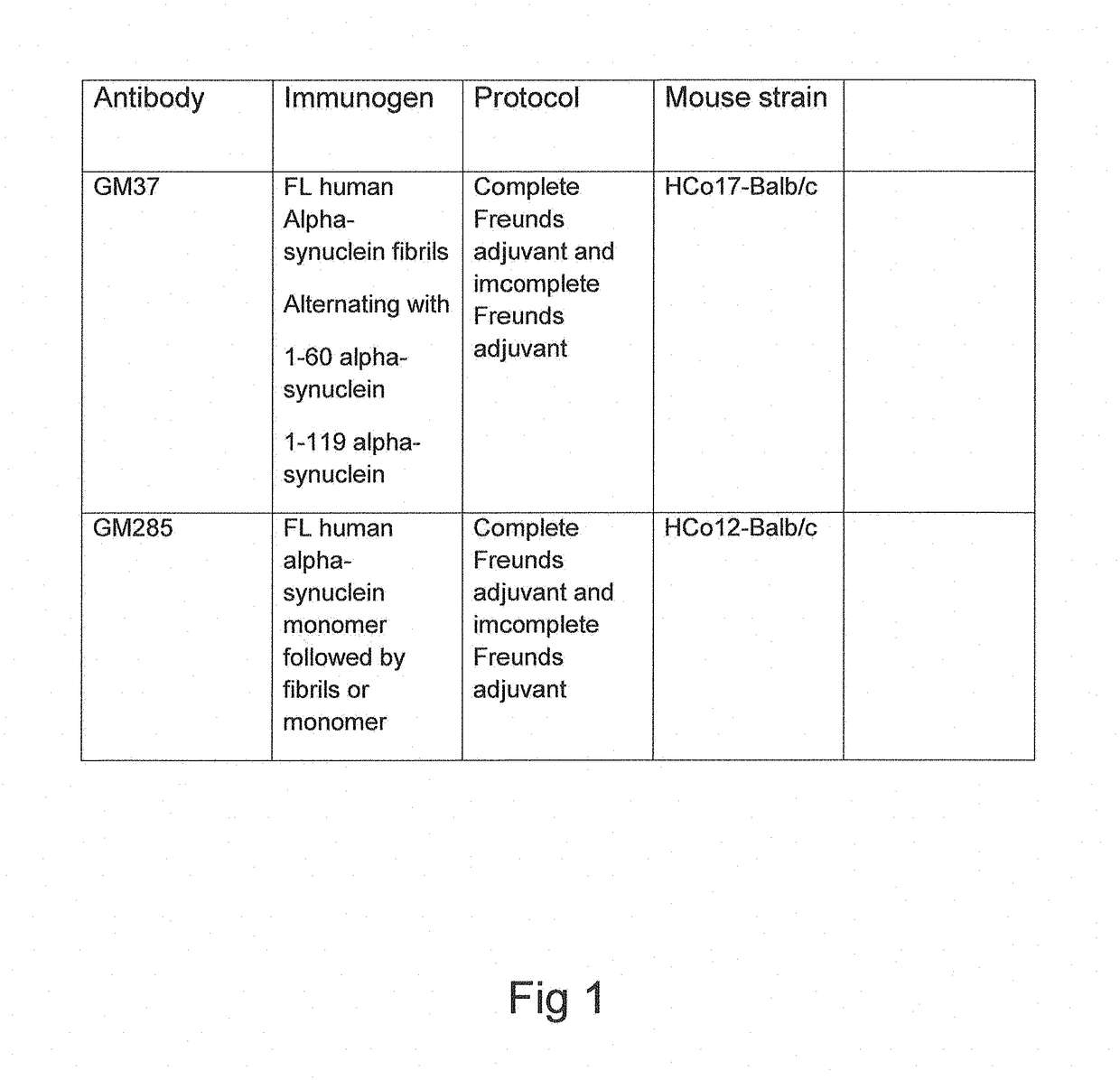
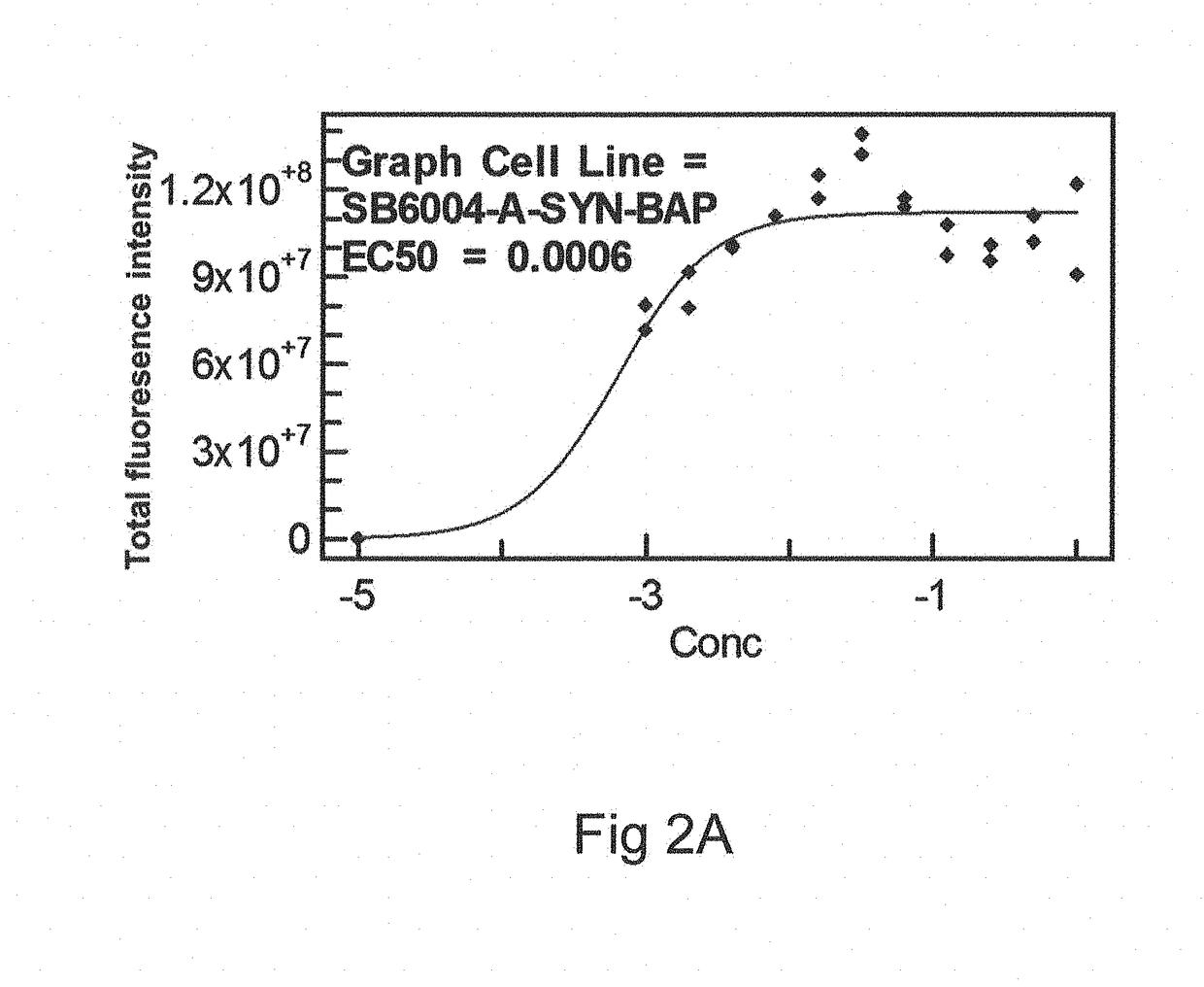
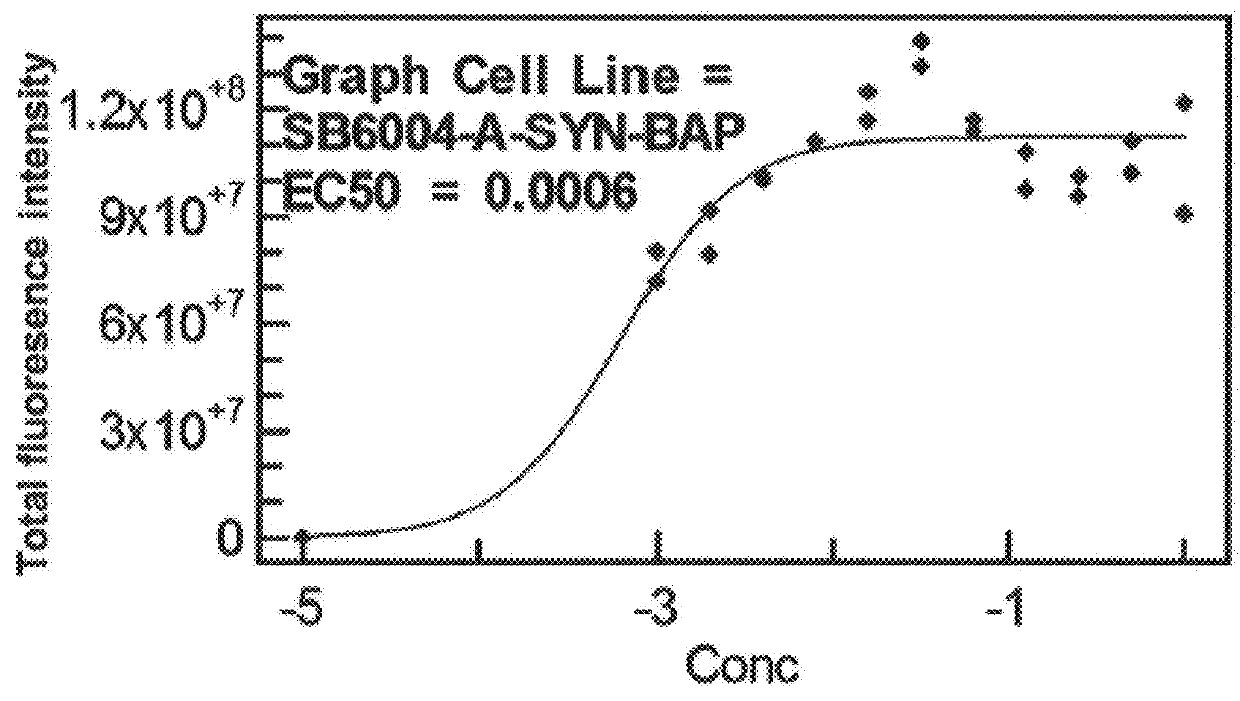
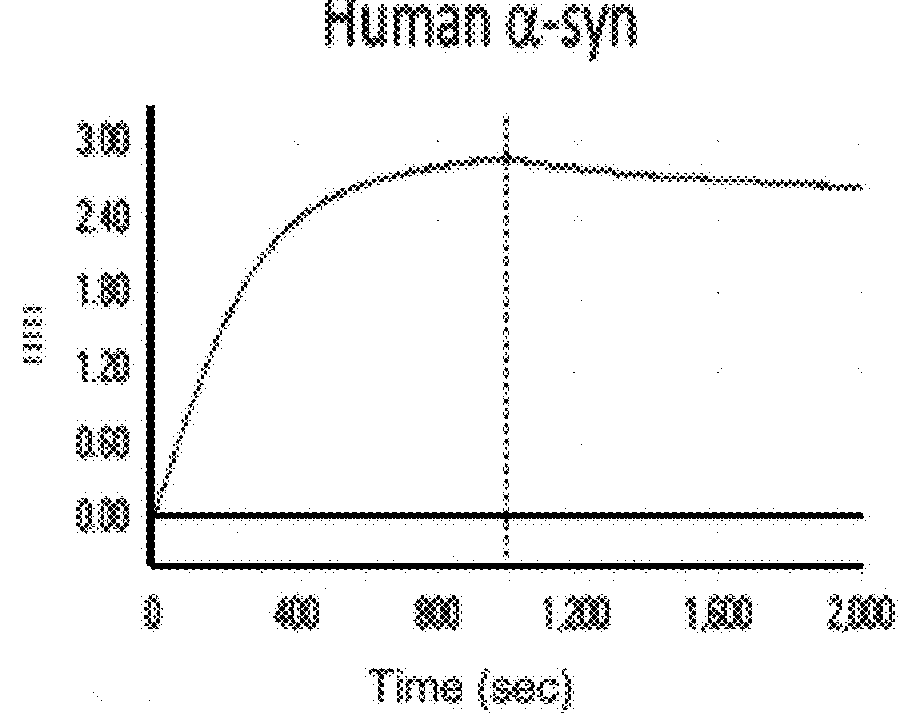
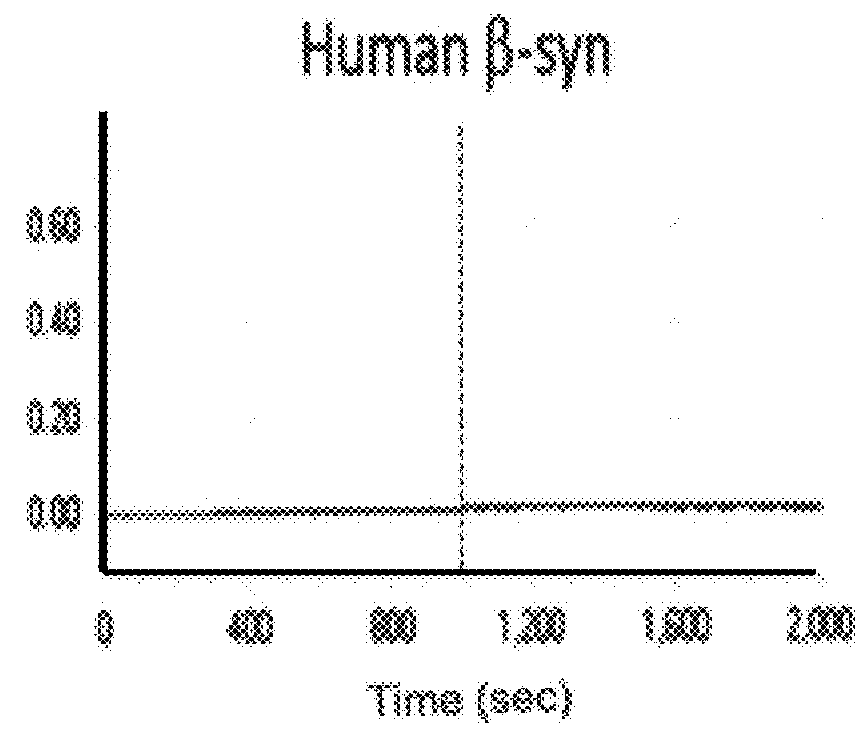
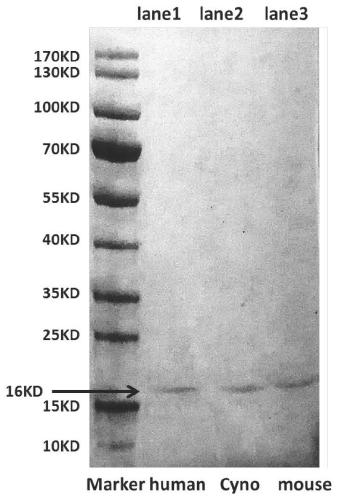
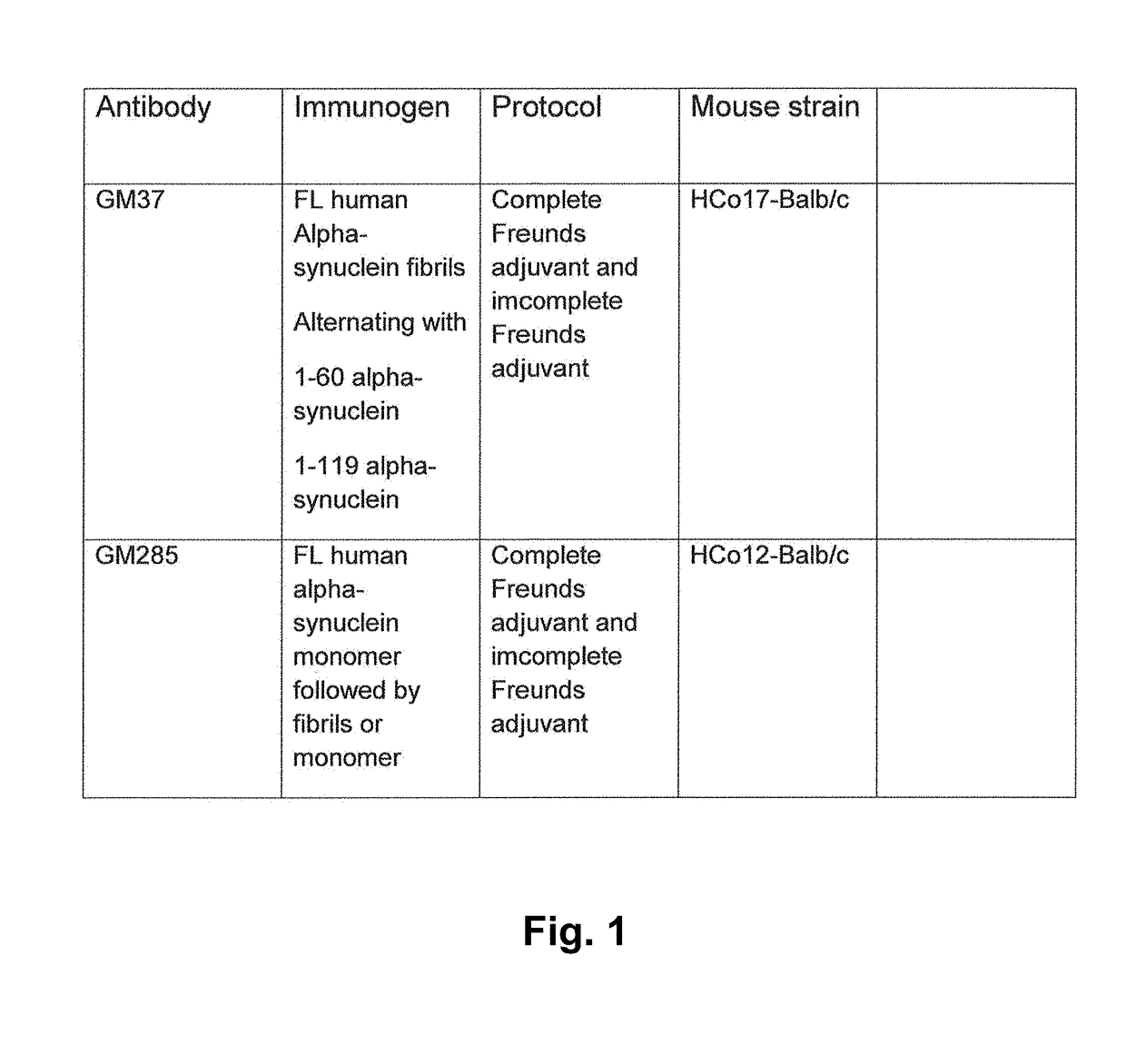
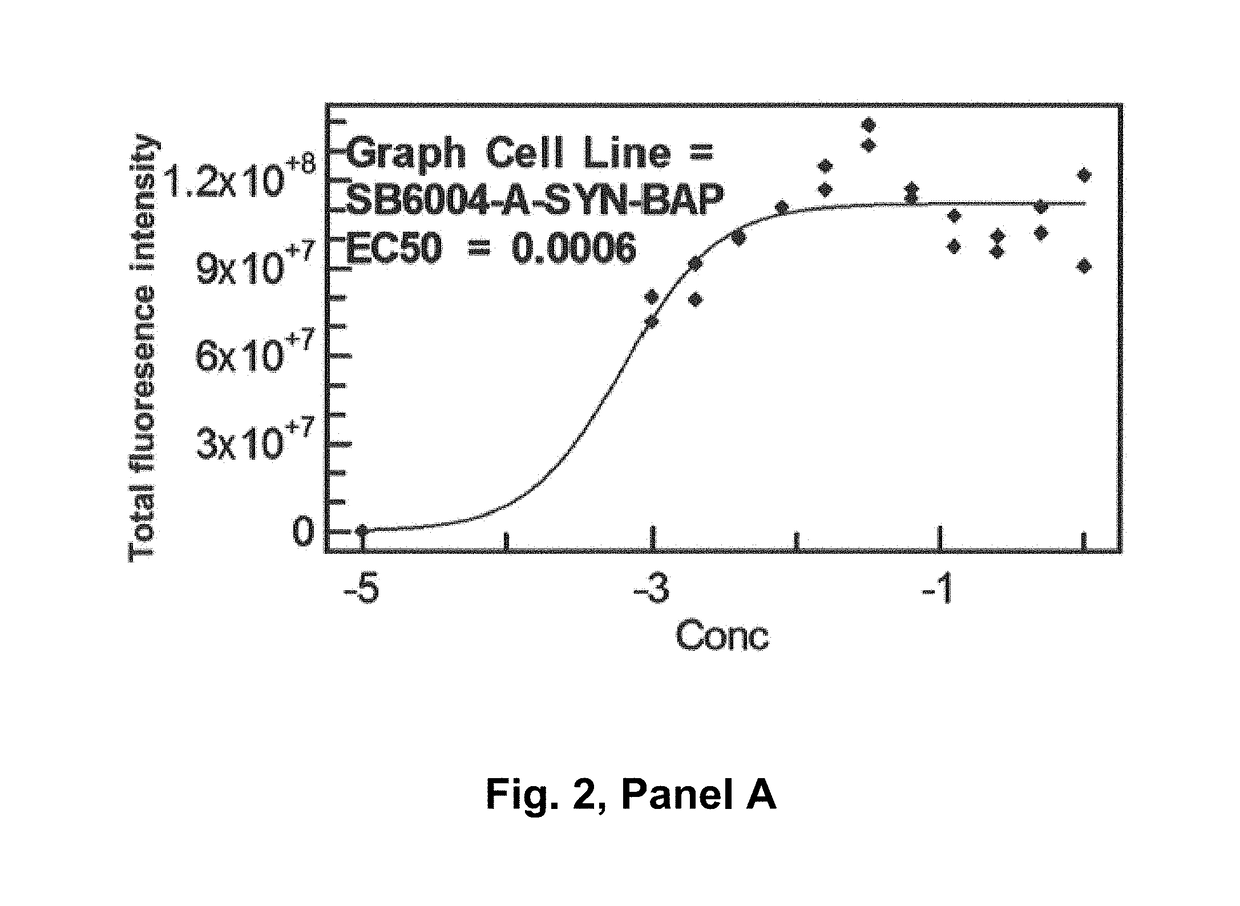
Patents
Literature
82 results about "Multisystem atrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Overview. Multiple system atrophy (MSA) is a rare, degenerative neurological disorder affecting your body's involuntary (autonomic) functions, including blood pressure, breathing, bladder function and muscle control. Formerly called Shy-Drager syndrome, MSA shares many Parkinson's disease-like symptoms, such as slow movement,...
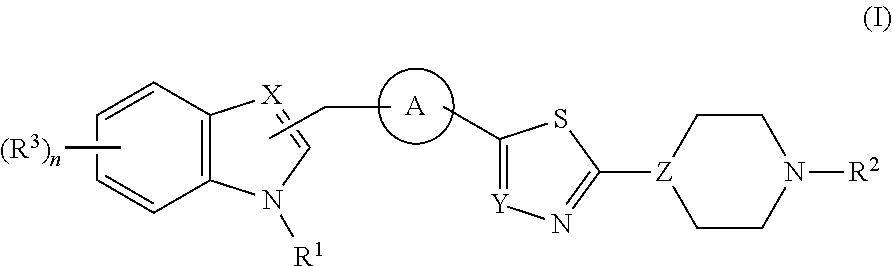
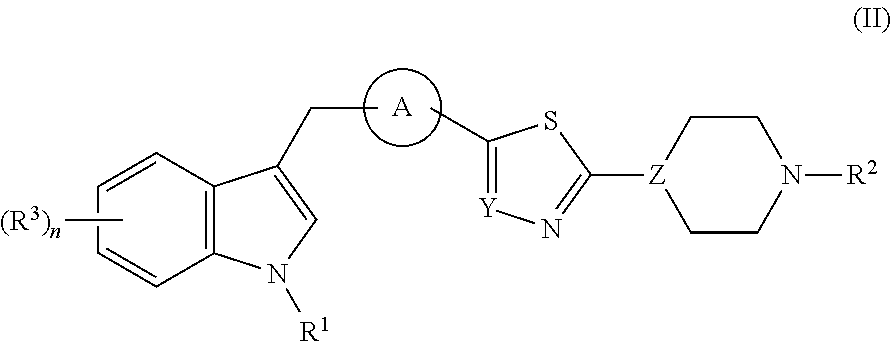
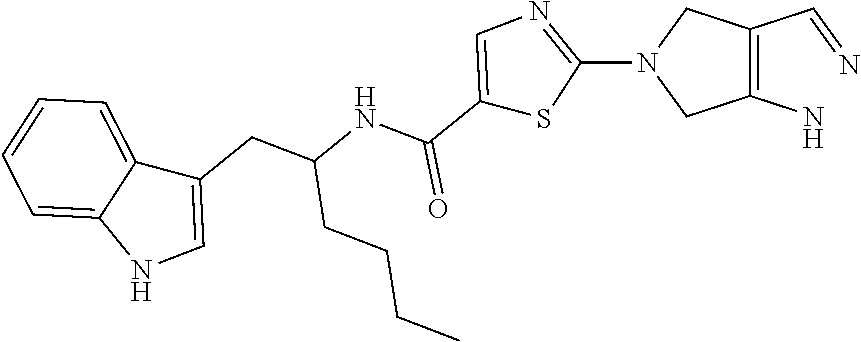
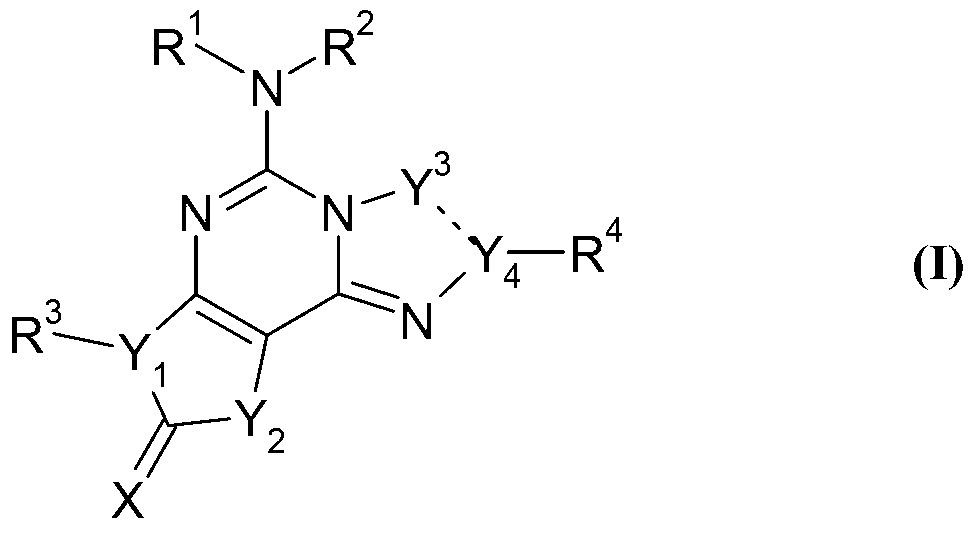
Thiadiazole derivatives for the treatment of neurodegenerative diseases
ActiveUS20090054410A1Counteract and inhibit toxic propertyEfficient methodBiocideNervous disorderSynucleinopathiesNiemann–Pick disease
This invention provides specifically substituted 1,2,4-thiadiazole derivatives for use in the treatment of an α-synucleopathy such as Parkinson's disease, diffuse Lewy body disease, traumatic brain injury, amyotrophic lateral sclerosis, Niemann-Pick disease, Hallervorden-Spatz syndrome, Down syndrome, neuroaxonal dystrophy, multiple system atrophy and Alzheimer's disease. This invention also provides various methods for producing such substituted 1,2,4-thiadiazole derivatives.
Owner:REMYND NV
Differential diagnosis of neurological diseases
InactiveUS6670137B2Nervous disorderPeptide/protein ingredientsDementia with Lewy bodiesNervous system
Owner:INNOGENETICS NV
Agents, Uses and Methods for the Treatment of Synucleinopathy
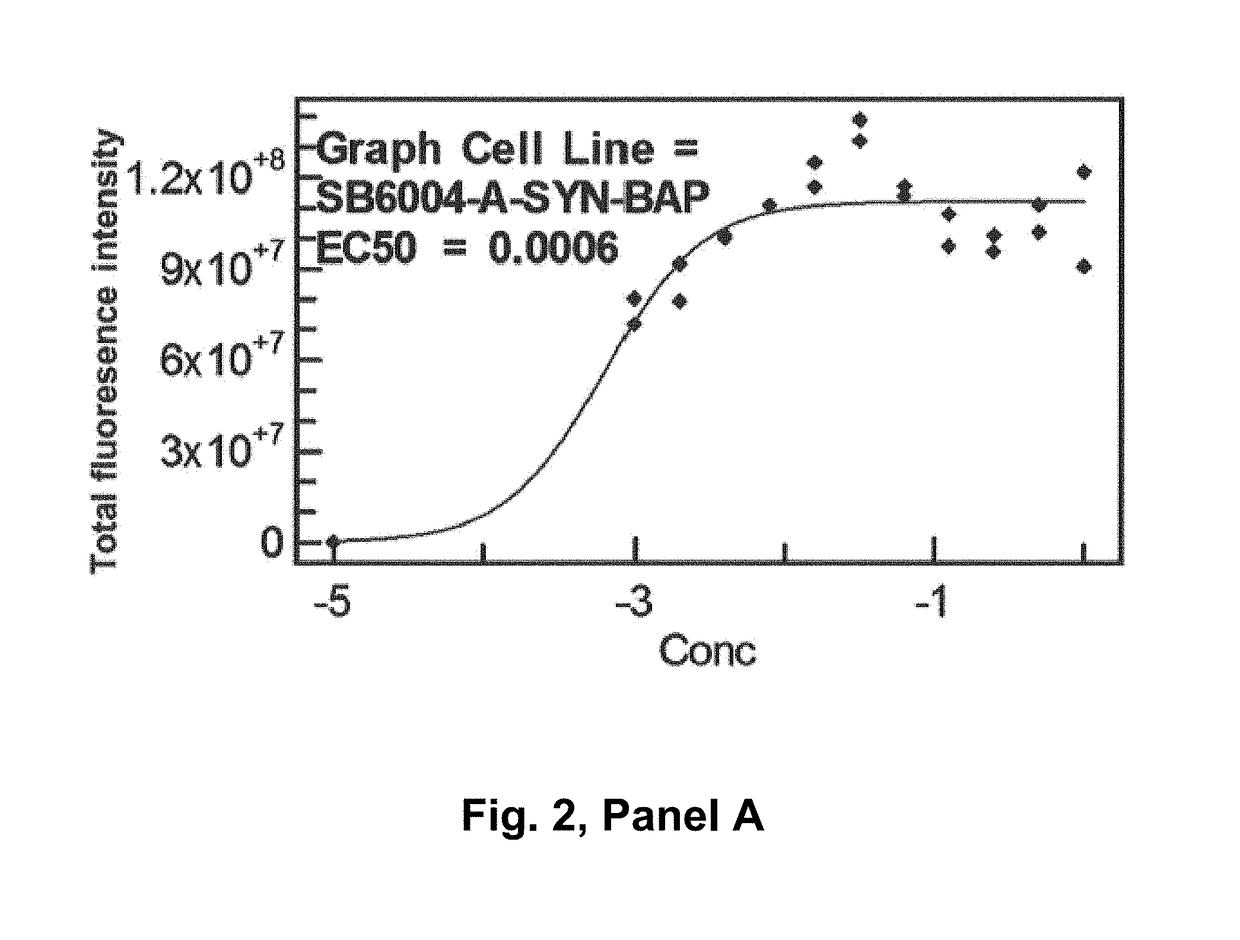
The invention relates to novel monoclonal anti-alpha-synuclein antibodies. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy.
Owner:H LUNDBECK AS
Agents, uses and methods for the treatment of synucleinopathy
The invention relates to combinational treatment using a monoclonal anti-alpha-synuclein antibody and an additional medicament. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy together with another medicament of the invention.
Owner:H LUNDBECK AS
Neurotrophic and neuroprotective peptides
InactiveUS20060036073A1Easy to derivatizeImprove solubilityNervous disorderPeptide/protein ingredientsAcute hypoxiaOrgan system
Novel peptides, whose individual components are L-amino acids or D-amino acids are used as active ingredients in medicaments for treating diseases in which the increased occurrence of free radicals plays a pathophysiological role, or for treating diseases involving acute hypoxia or ischaemia in an organ system of the body, in particular in the central nervous system, or for treating iron-storage diseases such as Hallervorden-Spatz syndrome, or for treating neurodegenerative diseases, in particular Alzheimer's disease, the Lewy body variant of Alzheimer's disease, Parkinson's disease, multi-system atrophy, Lewy body dementia or Huntington's chorea and all syndromes that are similar to the neurodegenerative diseases.
Owner:JSW RES FORSCHUNGSLABOR
Sirtuin 1 and the Treatment of Neurodegenerative Diseases
InactiveUS20110015272A1Urea derivatives preparationBiocideHuntingtons choreaDementia with Lewy bodies
This invention relates to bis- and tris-dihydroxyaryl compounds and their methylenedioxy analogs and pharmaceutically acceptable salts and their use in the modulation of Sirtuin 1 (Sirt1) and there use in neuroprotection for subject suffering from neurodegenerative diseases such as Alzheimer's disease, Huntington's disease, Amyotrophic lateral sclerosis, frontotemporal dementia, Parkinson's disease, including Parkinson's plus diseases such as multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration and dementia with Lewy bodies, and in the manufacture of medicaments for such Sirt1 modulation and neuroprotection.
Owner:PROTEOTECH
Agents, Uses and Methods for the Treatment of Synucleinopathy
ActiveUS20180179271A1Nervous disorderIn-vivo radioactive preparationsAlpha-synucleinAutonomic symptoms
The invention relates to novel monoclonal anti-alpha-synuclein antibodies. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy.
Owner:H LUNDBECK AS
Purpose of toluylene glycosides and its derivant for restraining synapse ribonucleoprtein excess expression
ActiveCN101161245AOrganic active ingredientsNervous disorderDementia with Lewy bodiesAlpha-synuclein
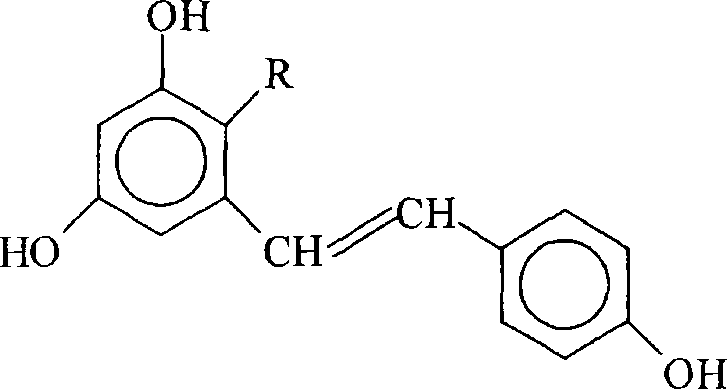
Application of stilbene glucoside or its derivative in inhibiting alpha-synuclein over expression is disclosed in the present invention. As the result of the test shows, stilbene glucoside or its derivative can effectively inhibit alpha-synuclein over expression, can be used for treating or preventing a plurality of nervous system diseases such as Parkinson's disease, dementia with Lewy body, simple autonomic nerve dysfunction, multiple system atrophy and etc. caused by alpha-synuclein over expression.
Owner:XUANWU HOSPITAL OF CAPITAL UNIV OF MEDICAL SCI
Agents, Uses and Methods for the Treatment of Synucleinopathy
ActiveUS20180127491A1Nervous disorderIn-vivo radioactive preparationsAtrophyAntiendomysial antibodies
The invention relates to novel monoclonal anti-alpha-synuclein antibodies. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy.
Owner:H LUNDBECK AS
Anti-alpha-synuclein monoclonal antibody and application thereof
ActiveCN110172098AFree from toxicityHigh affinityNervous disorderImmunoglobulins against animals/humansBeta-synucleinAutonomic symptoms
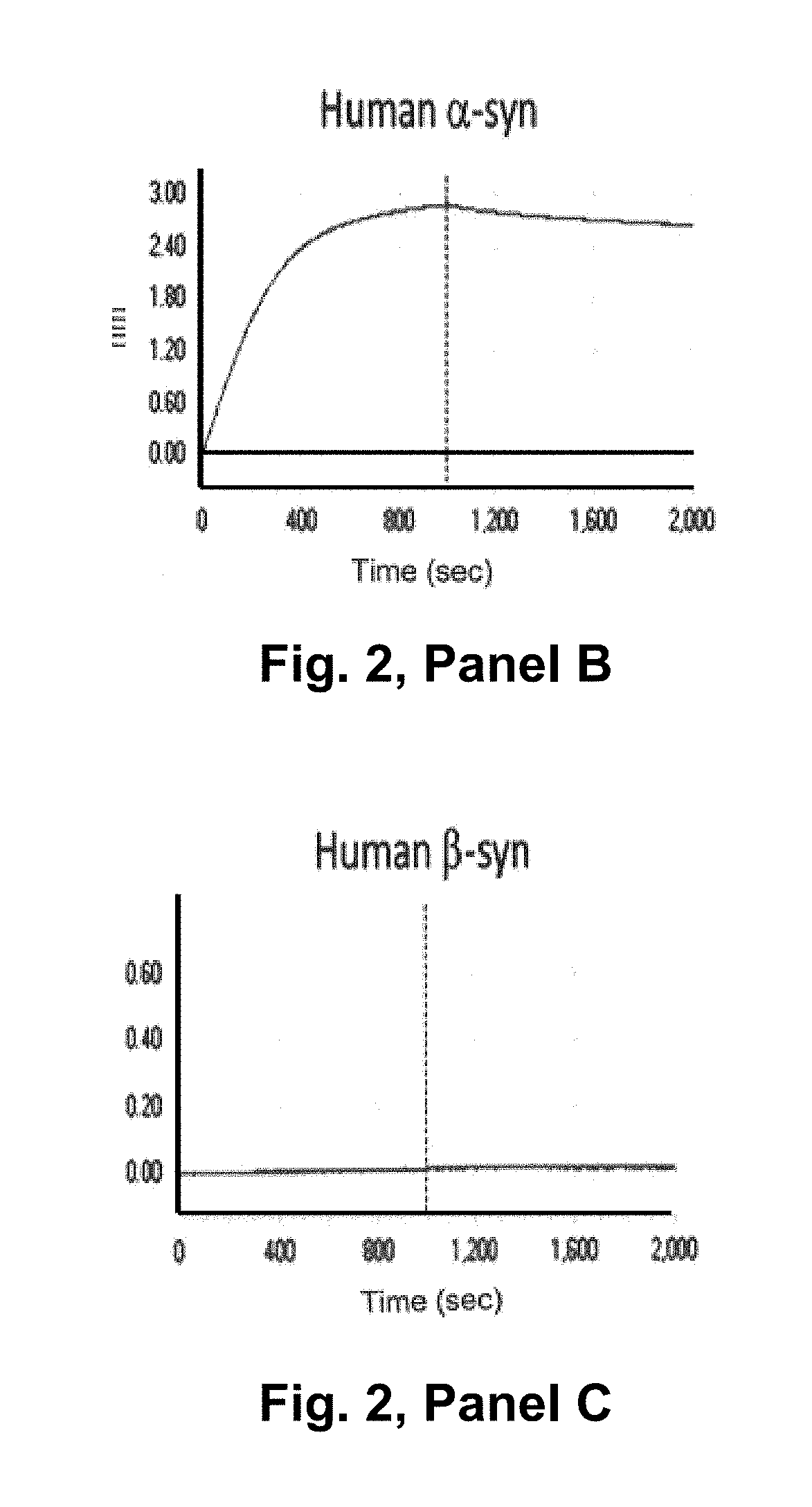
The invention relates to the technical field of antibody drugs, and specifically relates to an anti-alpha-synuclein monoclonal antibody and application thereof. The anti-alpha-synuclein monoclonal antibody provided by the invention can specifically bind to monomers and aggregates of alpha-synuclein, has high affinity to monomers and aggregates of a human alpha-synuclein, has no affinity to beta-synuclein and gamma-synuclein, can effectively inhibit the polymerization of the monomers of alpha-synuclein, promote microglial cells to clear formed alpha-synuclein aggregates, and protect neuronal cells from the toxicity of the alpha-synuclein aggregates, and can be used to prevent, treat and diagnose alpha-synuclein-related diseases and conditions such as Parkinson's disease, Lewy body dementia,Alzheimer's disease with Parkinson's disease, pure autonomic failure, and multiple system atrophy.
Owner:CHANGCHUN UNIV OF TECH
Agents, Uses and Methods for the Treatment of Synucleinopathy
The invention relates to novel monoclonal anti-alpha-synuclein antibodies. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy.
Owner:H LUNDBECK AS
Glycolipids as treatment for disease
InactiveUS20120035120A1High affinityExtended half-lifeBiocideNervous disorderDiseaseHuntingtons chorea
This invention provides compounds, compositions, and methods for treating a disorder selected from cancer, hyperinsulinemia, hypoglycemia, hyperinsulinemia with hypoglycemia, atypical Parkinson's disease, Huntington's disease, multiple systems atrophy, GM3 synthase deficiency, GM2 synthase deficiency or tauopathy.
Owner:SENEB BIOSCI
Di- and tri-heteroaryl derivatives as inhibitors of protein aggregation
The present invention relates to certain di- and tri-heteroaryl derivatives, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, and multiple system atrophy.
Owner:UCB PHARMA SRL
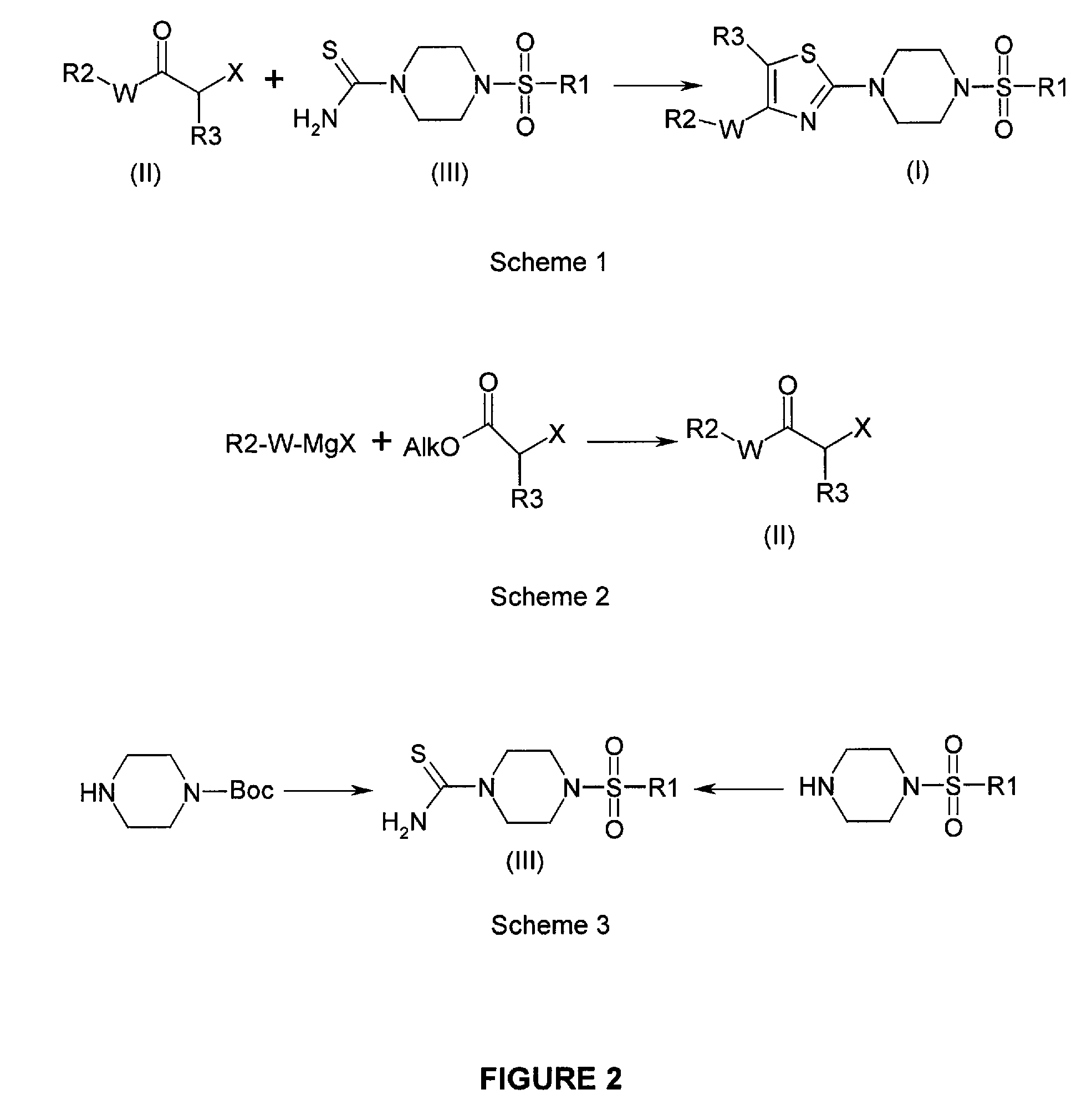
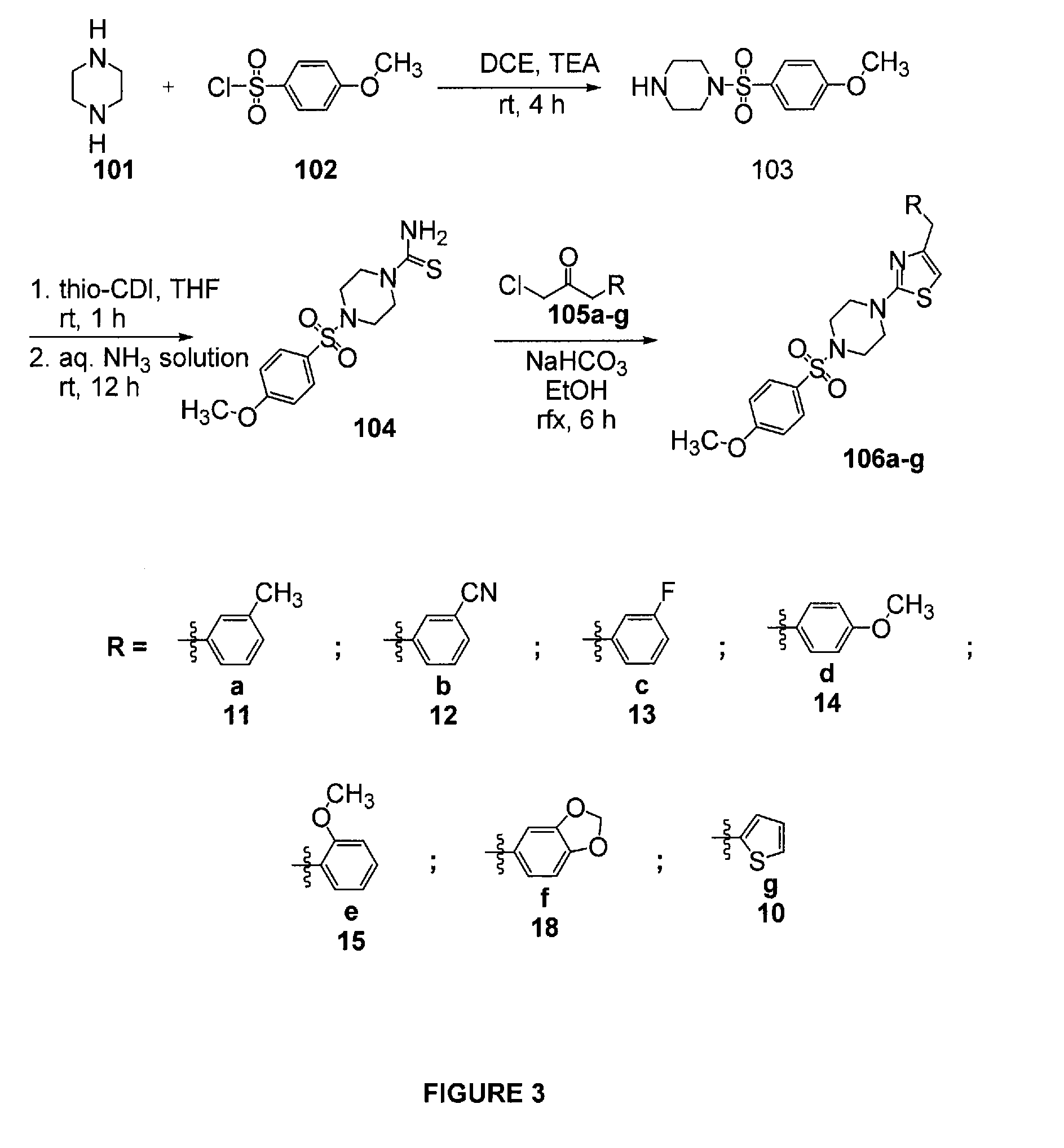
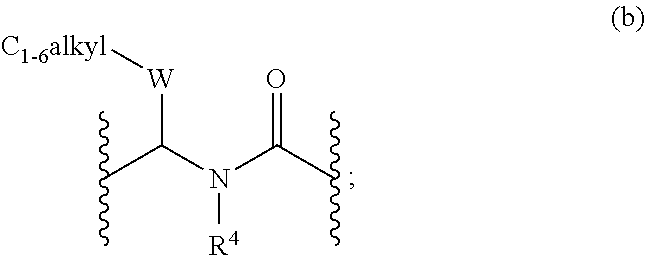
N-sulfonyl thiazolylpiperazine derivatives and related n-sulfonyl heterocyclic derivatives for the treatment of neuro degenerative diseases
ActiveUS20100197703A1Organic active ingredientsNervous disorderNeuro-degenerative diseaseBULK ACTIVE INGREDIENT
This invention provides thiazolylpiperazine derivatives, and N-sulfonyl heterocyclic derivatives including phenyl- and benzyl-thiazolylpiperidine derivatives, and pharmaceutically acceptable salts thereof, which are useful active ingredients for administration in a method for the treatment of an α-synucleopathy such as Parkinson's disease, diffuse Lewy body disease, traumatic brain injury, amyotrophic lateral sclerosis, Niemann-Pick disease, Hallervorden-Spatz syndrome, Down syndrome, neuroaxonal dystrophy, multiple system atrophy and Alzheimer's disease. This invention also provides methods for making such derivatives, and pharmaceutical compositions including such derivatives together with pharmaceutically acceptable excipients.
Owner:REMYND NV
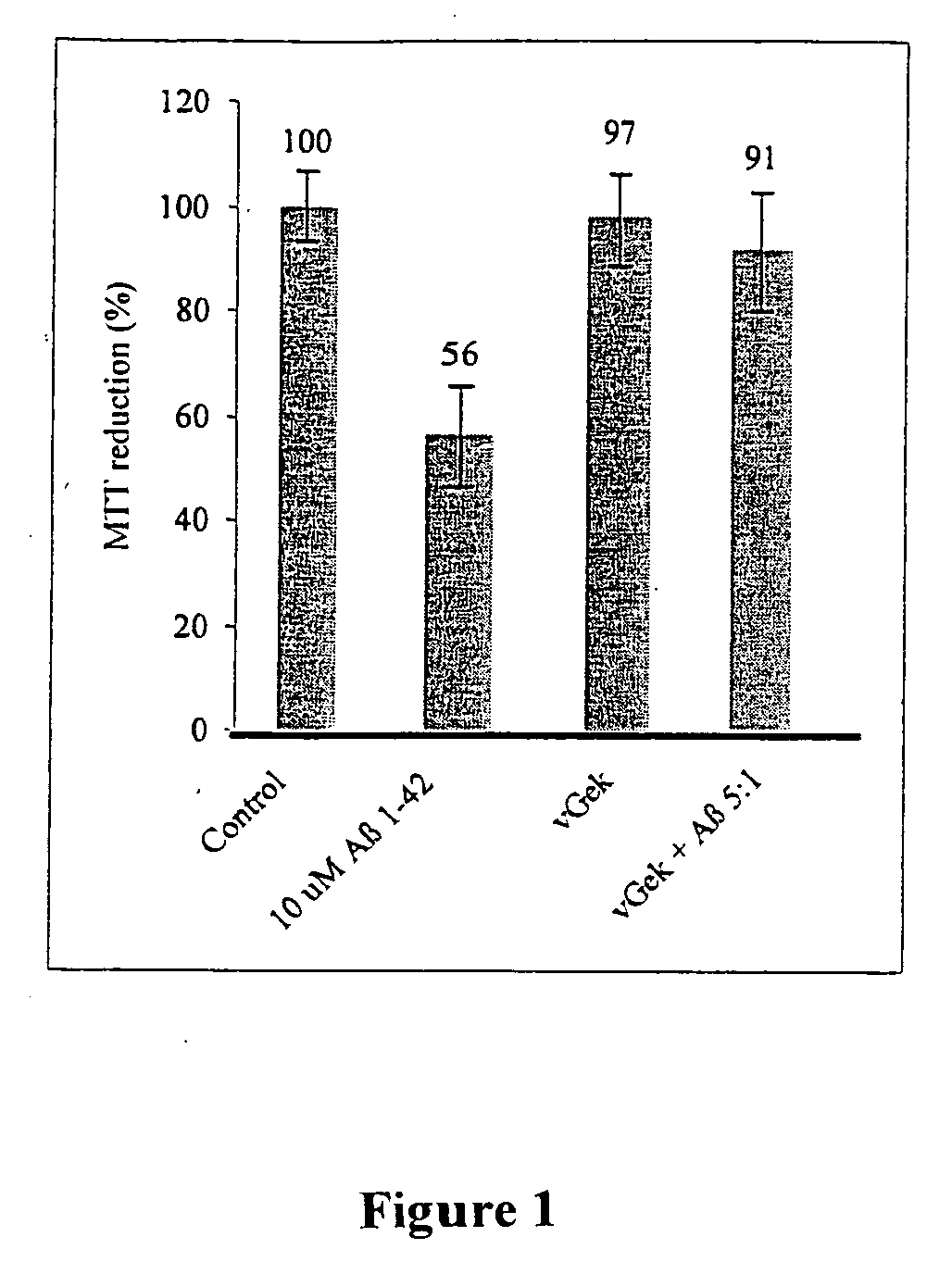
Peptidomimetic agents from dextrorotatory amino acids as well as pharmaceutical agents that contain the latter for treatment of neurodegenerative diseases
A peptidomimetic agent from dextrorotatory amino acids includes vGek with Dval-gly-Dglu-Dlys as a central D-amino acid sequence, whereby gly is equal to D-glycine, which is equal to L-glycine. Pharmaceutical agents for use in the treatment of neurodegenerative diseases, in particular Alzheimer's disease, Parkinson's disease, Lewy Body dementia, Creutzfeldt-Jakob disease, as well as Huntington's Chorea disease, multi-system atrophy as well as disorders similar to these neurodegenerative diseases that contain at least one peptidomimetic agent from dextrorotatory amino acids are also included.
Owner:JSW RES FORSCHUNGSLABOR
Di- and tri-heteroaryl derivatives as inhibitors of protein aggregation
The present invention relates to certain di- and tri-heteroaryl derivatives, pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, and multiple system atrophy.
Owner:UCB PHARMA SRL
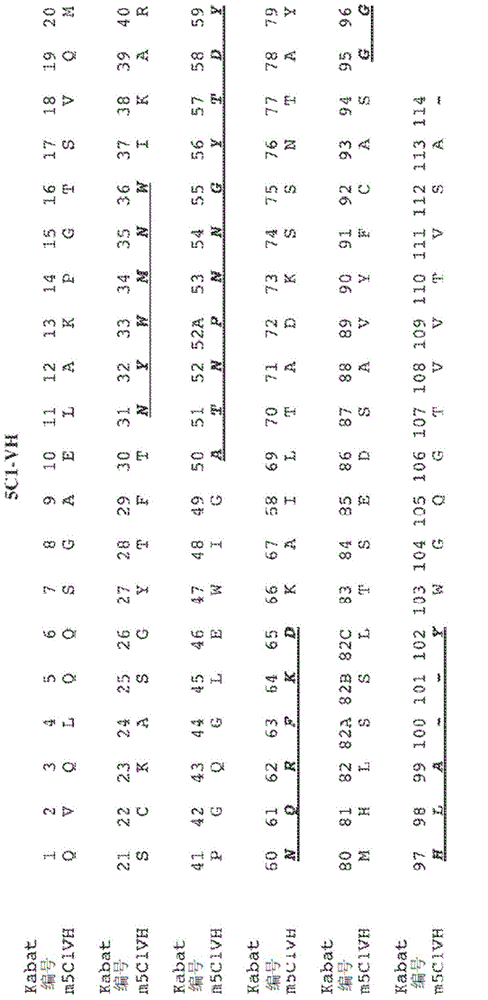
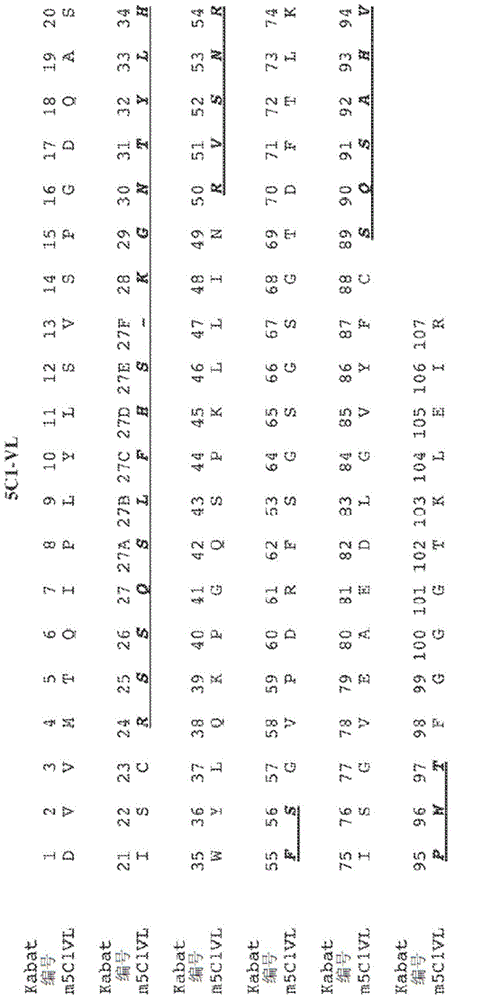
Antibodies recognizing alpha-synuclein
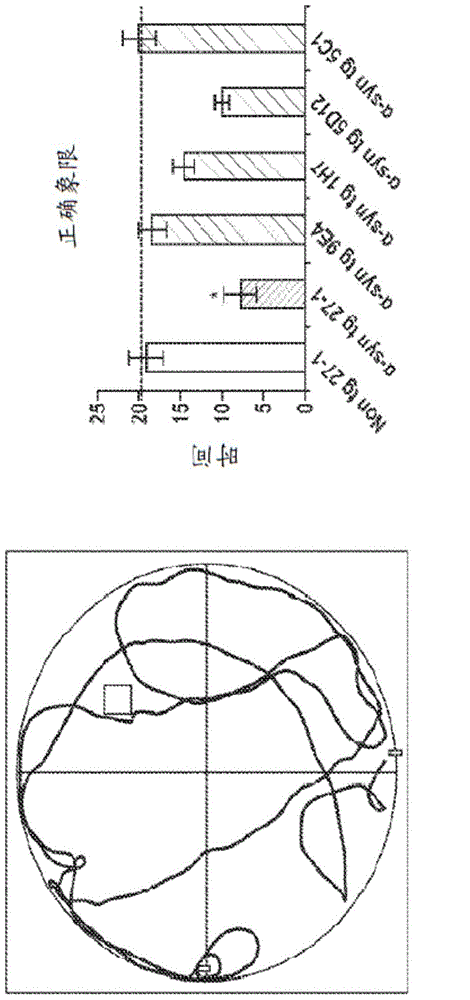
The invention provides monoclonal antibody 5C1 and related antibodies. The 5C1 antibody binds to an epitope within residues 118-126 of α-synuclein. The antibodies of the invention are useful, for example, for treating and / or diagnosing disorders associated with α-synuclein, particularly accumulation of α-synuclein deposits. Such disorders include Lewy body diseases, such as Parkinson's disease, Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy (MSA).
Owner:PROTHENA BIOSCI LTD
Agents, uses and methods for the treatment of synucleinopathy
The invention relates to combinational treatment using a monoclonal anti-alpha-synuclein antibody and an additional medicament. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy together with another medicament of the invention.
Owner:H LUNDBECK AS
Bis-heteroaryl derivatives as modulators of protein aggregation
ActiveUS20190367502A1Improvement in sensorimotorIncrease the number ofOrganic active ingredientsNervous disorderHuntingtons choreaAtrophy
The present invention relates to bis-heteroaryl compounds of formula (I), pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto-temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
Owner:UCB PHARMA SRL
NMDA NR2B antagonists for treatment
The invention provides new methods for treating certain disorders resulting from neurodegeneration and for treating depression which comprise administration of NR2B subunit selective NMDA antagonists. The disorders that can be treating by the invention include hearing loss, vision loss, neurodegeneration caused by epileptic seizures, neurotoxin poisoning, Restless Leg Syndrome, multi-system atrophy, non-vascular headache, and depression.
Owner:PFIZER INC +1
Bicyclic bis-heteroaryl derivatives as modulators of protein aggregation
The present invention relates to certain bicyclic bis-heteroaryl compounds of Formula (I), pharmaceutical compositions containing them, and methods of using them, including methods for preventing, reversing, slowing, or inhibiting protein aggregation, and methods of treating diseases that are associated with protein aggregation, including neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, Lewy body disease, Parkinson's disease with dementia, fronto-temporal dementia, Huntington's Disease, amyotrophic lateral sclerosis, and multiple system atrophy, and cancer including melanoma.
Owner:UCB PHARMA SRL
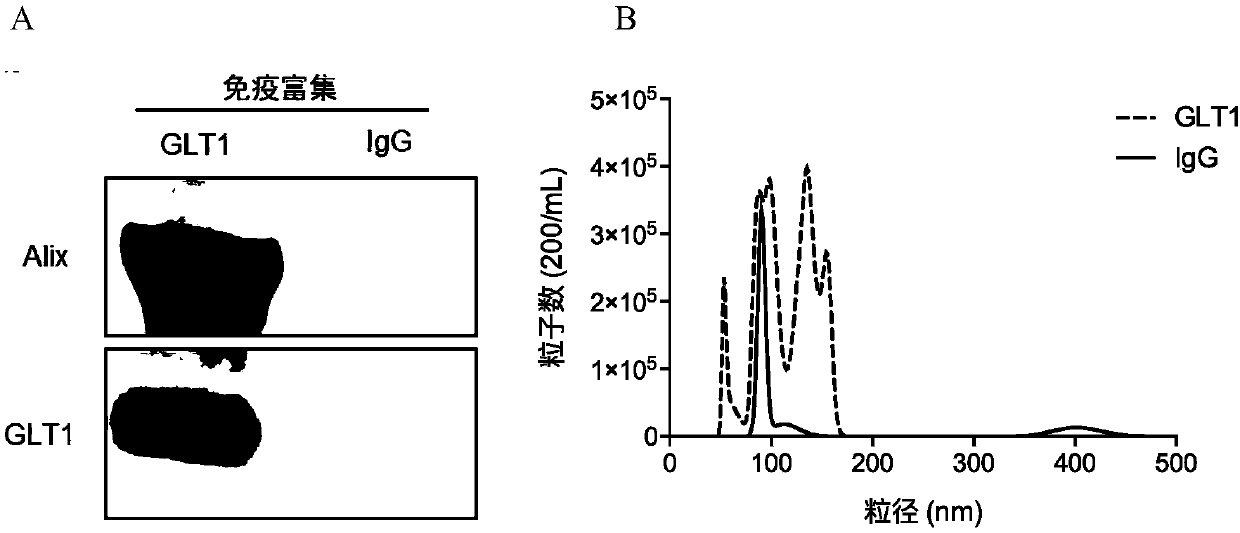
Method for enriching or detecting astrocyte-derived exosomes from biological fluids
InactiveCN110133272ARelieve painSuitable for routine clinical applicationDisease diagnosisBiological testingNervous systemImmune complex deposition
The invention discloses a method for enriching or detecting astrocyte-derived exosomes from biological fluids. The method for enriching the astrocyte-derived exosomes comprises the steps of (a) contacting a biological fluid containing an astrocyte-derived exosome with an anti-GLT1 antibody to form an immune complex, wherein the biological fluid is one or more selected from blood, serum, plasma, saliva and urine; and (b) enriching the astrocyte-derived exosome through the immune complex by adoption of a solid-phase or liquid-phase method. A biomarker capable of detecting the astrocyte-derived exosomes is used for assisted diagnosis, differential diagnosis and monitoring of central nervous system diseases such as Alzheimer's disease, Parkinson's disease, multiple system atrophy, prion disease and nervous system tumors such as gliomas and the like, so that markers are prevented from being obtained through traumatic operations.
Owner:PEKING UNIV
Alpha synuclein toxicity
InactiveUS20120014964A1Suppressing the endonuclease G activityLow toxicityOrganic active ingredientsNervous disorderDementia with Lewy bodiesApoptosis
Present inventions demonstrates that alpha synuclein toxicity such as α-synuclein mediated cell death, alpha synuclein induced reactive oxygen species (ROS) in a cell requires the proapoptotic endonuclease G and that the deletion of the endonuclease G or suppressing of the endonuclease G apoptotic pathway attenuates or counteracts such alpha synuclein toxicity. The present invention compositions and methods for inhibition of α-synuclein toxicity. The inhibiting α-synuclein toxicity can be used in methods of treatment of synucleinopathies, such as Parkinson's disease (PD), dementia with Lewy bodies (DLB), pure autonomic failure (PAF), and multiple system atrophy (MSA) and the manufacture of medicaments for such treatment. In particular The subject matter provided in herein relates to a pharmaceutical compositions containing inhibitors of endonuclease G, and their use in the treatment of synucleinopathies, such as Parkinson's disease, dementia with Lewy bodies, pure autonomic failure, and multiple system atrophy and the manufacture of medicaments for such treatment. Furthermore the present invention relates to a method for the identification of compounds attenuating the synuclein toxicity, said method comprising evaluating the inhibitory action of said compound on the endonuclease G dependent apoptosis.
Owner:KATHOLIEKE UNIV LEUVEN +1
Fused tricyclic compounds as adenosine receptor antagonist
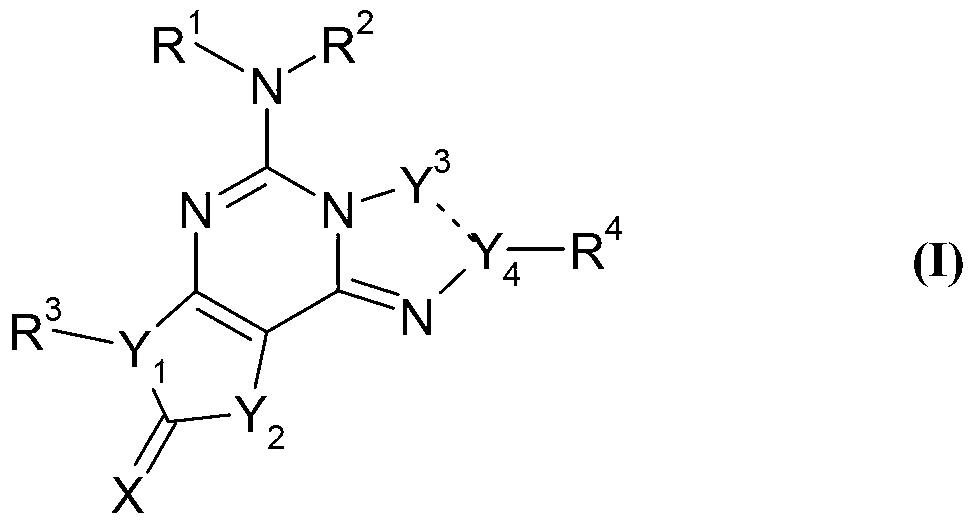
InactiveCN103261202AAntibacterial agentsSenses disorderVasopressin AntagonistsAdenosine a2a receptors
The present disclosure relates to fused tricyclic compounds of formula (I) or its tautomers, polymorphs, stereoisomers, prodrugs, solvate or a pharmaceutically acceptable salts, or pharmaceutical compositions containing them and methods of treating conditions and diseases that are mediated by thereof as A2A adenosine receptor antagonists. [Formula should be inserted here] The compounds of the present disclosure are useful in the treatment, prevention or suppression of diseases and disorders that may be susceptible to improvement by the mediation of adenosine A2A receptor.; Such conditions include, but are not limited to, Parkinsons disease, restless leg syndrome, Alzheimers disease, neurodegenerative disorder, inflammation, wound healing, dermal fibrosis, nocturnal myoclonus, cerebral ischaemia, myocardial ischemia, Huntington's disease, multiple system atrophy, corticobasal degeneration, Wilson's disease or other disorders of basal ganglia which results in dyskinesias, post traumatic stress disorder, hepatic cirrhosis, sepsis, spinal cord injury, retinopathy, hypertension, social memory impairment, depression, neuroprotection, narcolepsy or other sleep related disorders, attention deficit hyperactivity disorder, drug addiction, post traumatic stress disorder and vascular injury and the like. The present disclosure also relates to methods for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:ADVINUS THERAPEUTICS PVT LTD
NMDA NR2B antagonists for treatment
InactiveUS7019016B2Avoid nerve damageInhibition is effectiveBiocideSenses disorderAtrophyVascular headache
The invention provides new methods for treating certain disorders resulting from neurodegeneration and for treating depression which comprise administration of NR2B subunit selective NMDA antagonists. The disorders that can be treating by the invention include hearing loss, vision loss, neurodegeneration caused by epileptic seizures, neurotoxin poisoning, Restless Leg Syndrome, multi-system atrophy, non-vascular headache, and depression.
Owner:PFIZER INC +1
Quinolinone farnesyl transferase inhibitors for the treatment of synucleinopathies and other indications
InactiveUS20090253655A1Low toxicityReduce accumulationBiocideTissue cultureSynucleinopathiesFarnesyl Transferase Inhibitor
Novel quinolinone farnesyl transferase inhibitors are provided. These new compounds are useful in the treatment or prevention of synucleinopathies, such as Parkinson's Disease, Diffuse Lewy Body Disease, multiple system atrophy, and disorders of brain iron concentration including pantothenate kinase-associated neurodegeneration (e.g., PANK1), or other neurodegenerative / neurological diseases. Provided compounds are also useful in the treatment of proliferative diseases such as cancer, and in the treatment of neurological diseases, such as cognitive impairment, depression, and anxiety.The treatment including administering to a subject a therapeutically effective amount of an inventive farnesyl transferase inhibitor compound.
Owner:ASTRAZENECA AB
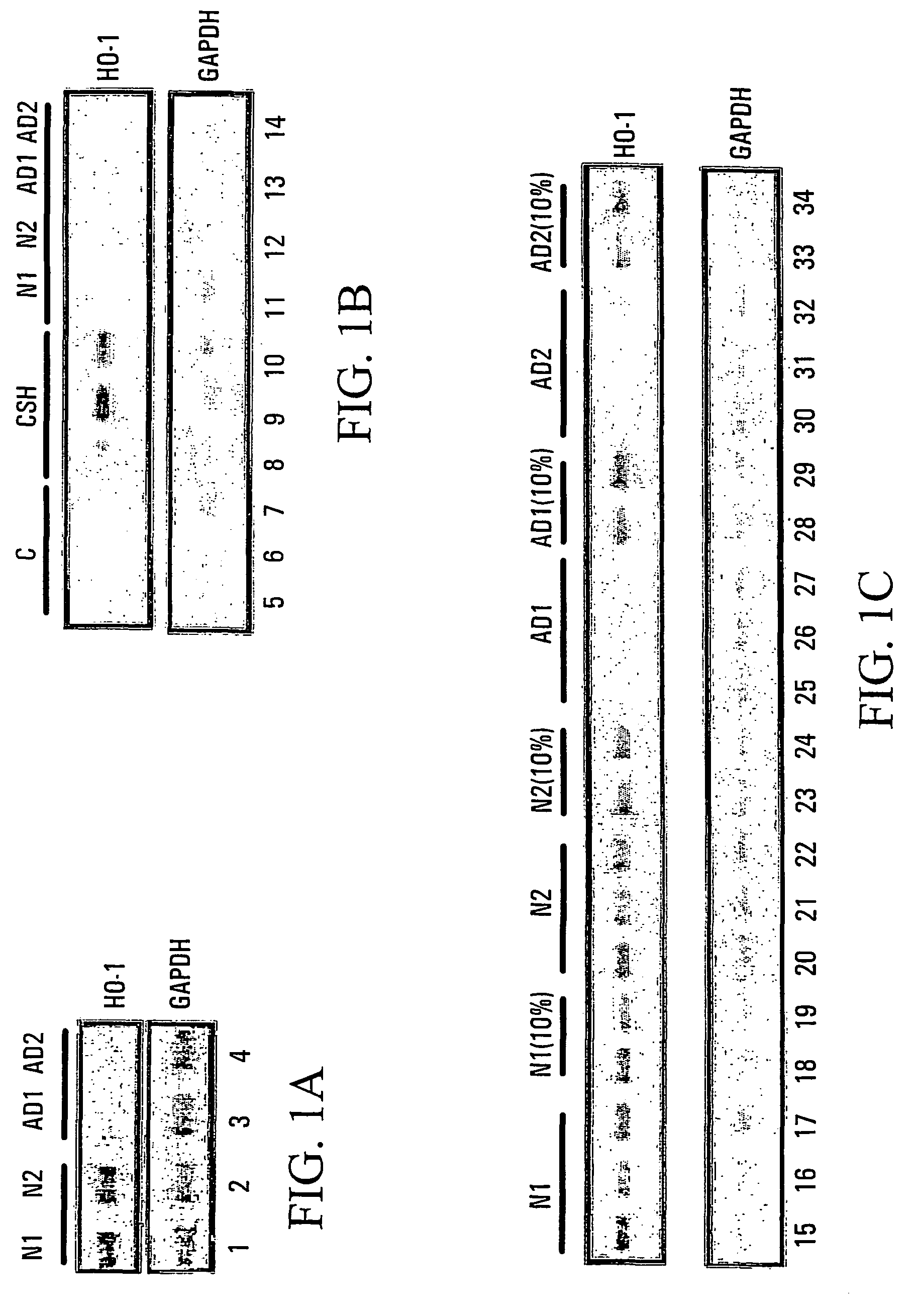
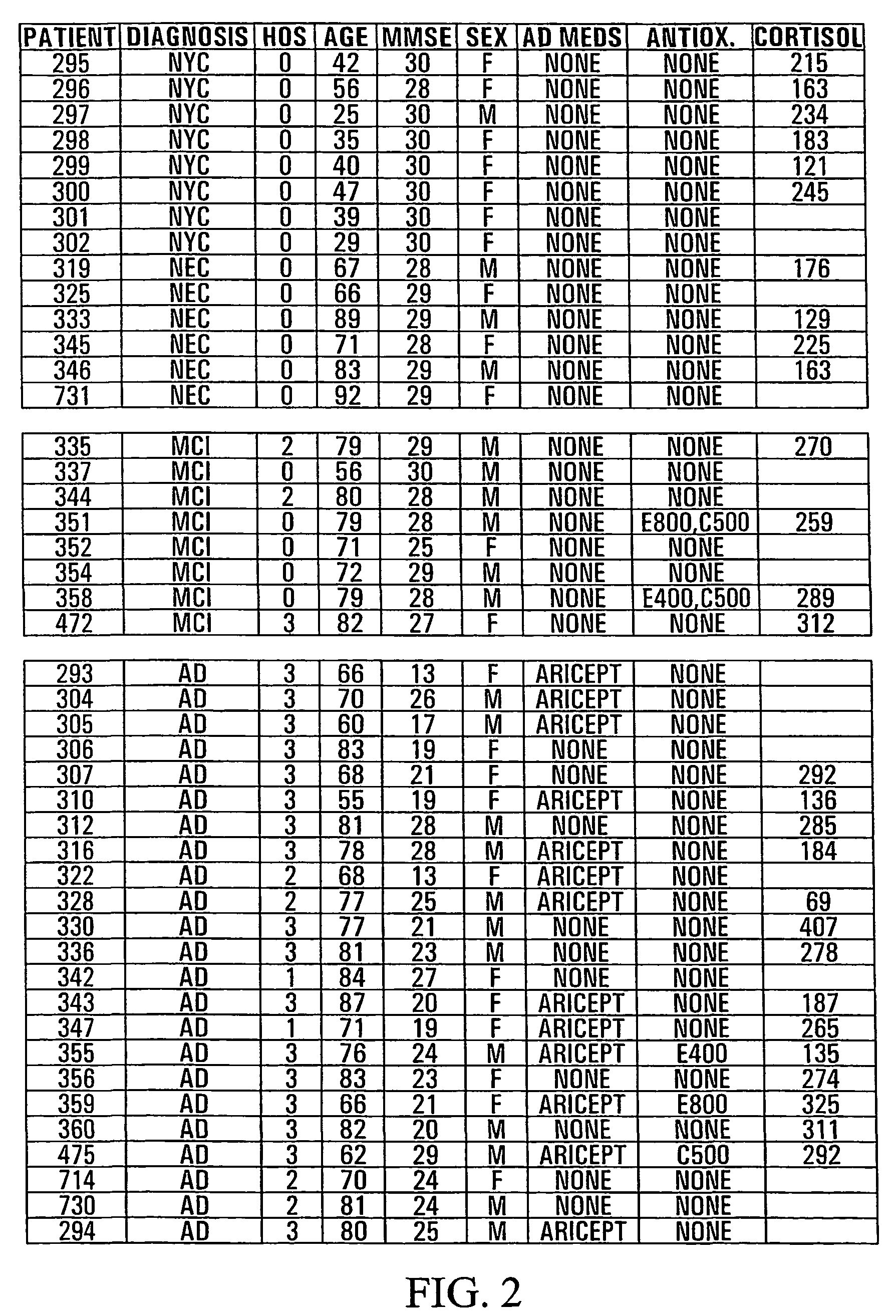
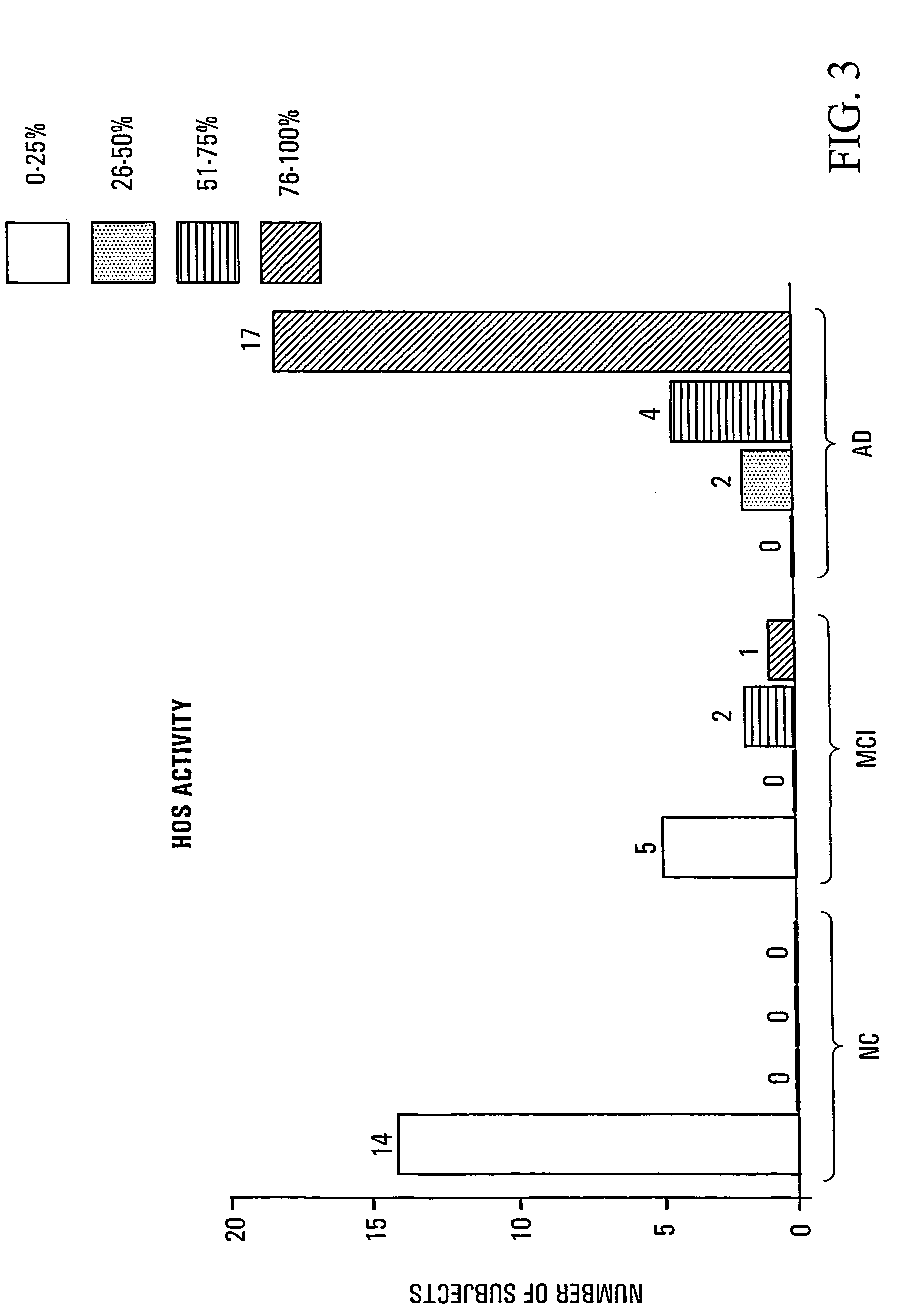
HO-1 suppressor as a diagnostic and prognostic test for dementing diseases
InactiveUS7105485B2Help studyEasy to manageAntibacterial agentsNervous disorderSevere hypothyroidismS syndrome
The invention relates to an improved method for predicting the onset of, diagnosing, prognosticating and / or treating dementing diseases. The method comprises determining the level of heme oxygenase-1 suppressor (HOS) activity and / or factor in tissue or body fluid obtained from a patient, and comparing said level with the corresponding level of HOS activity and / or factor in corresponding tissue or body fluid obtained from at least one control person. The tissue or body fluid is suitably blood, plasma, lymphocytes, cerebrospinal fluid, urine, saliva, epithelia or fibroblasts. The method is useful where the dementing disease is any of Alzheimer Disease, Age-Associated Cognitive Decline, Mild Cognitive Impairment, Parkinson disease with dementia, Progressive Supranuclear Palsy, Vascular (i.e. multi-infarct) Dementia, Lewy Body Dementia, Huntington's Disease, Down's syndrome, normal pressure hydrocephalus, corticobasal ganglionic degeneration, multisystem atrophy, head trauma, neurosyphilis, Creutzfeld-Jacob disease and other prion diseases, HIV and other encephalitides, and metabolic disorders such as hypothyroidism and vitamin B12 deficiency. The method may also prove useful in differentiating the“pseudodementia” of depression from Alzheimer disease.
Owner:SIR MORTIMER B DAVIS JEWISH GEN HOSPITAL
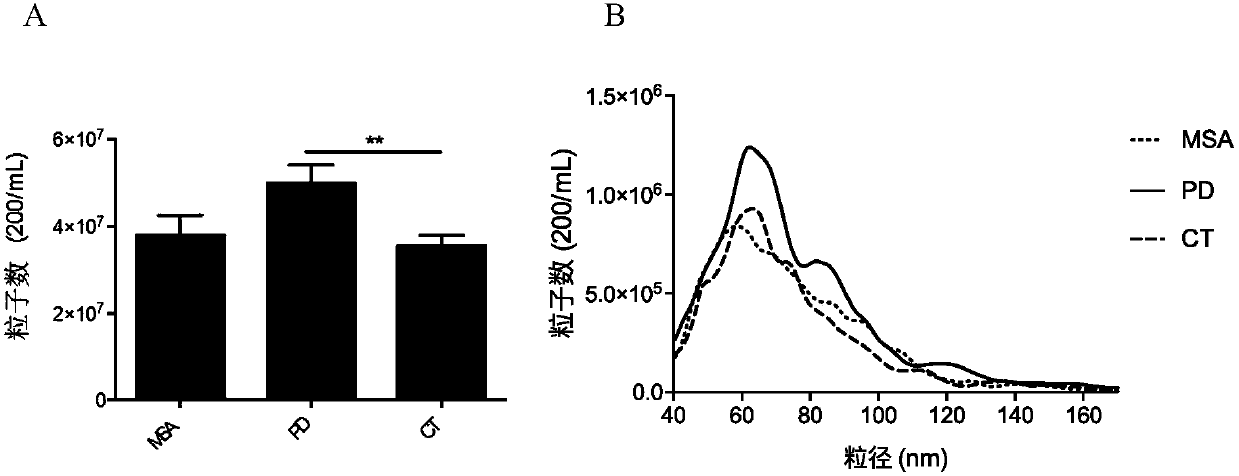
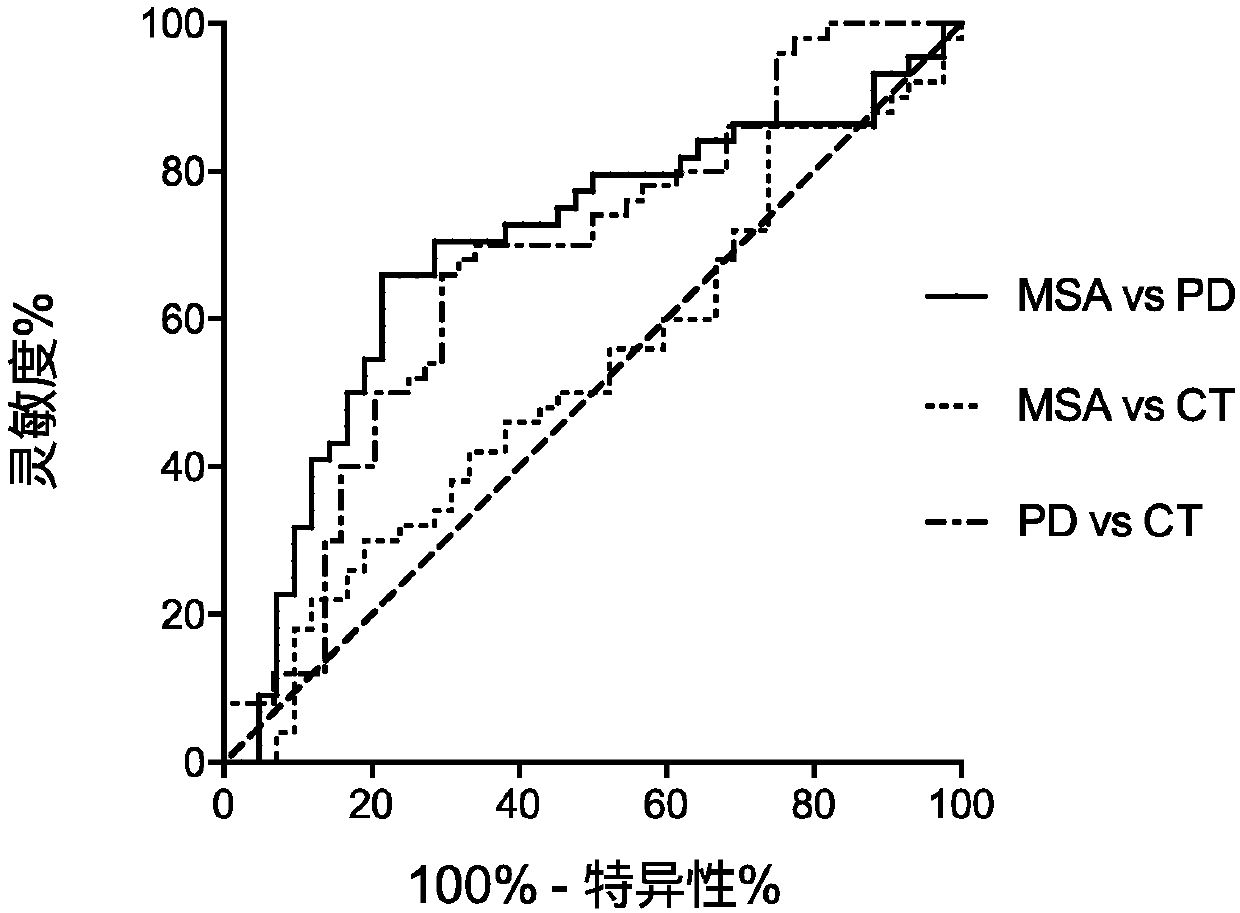
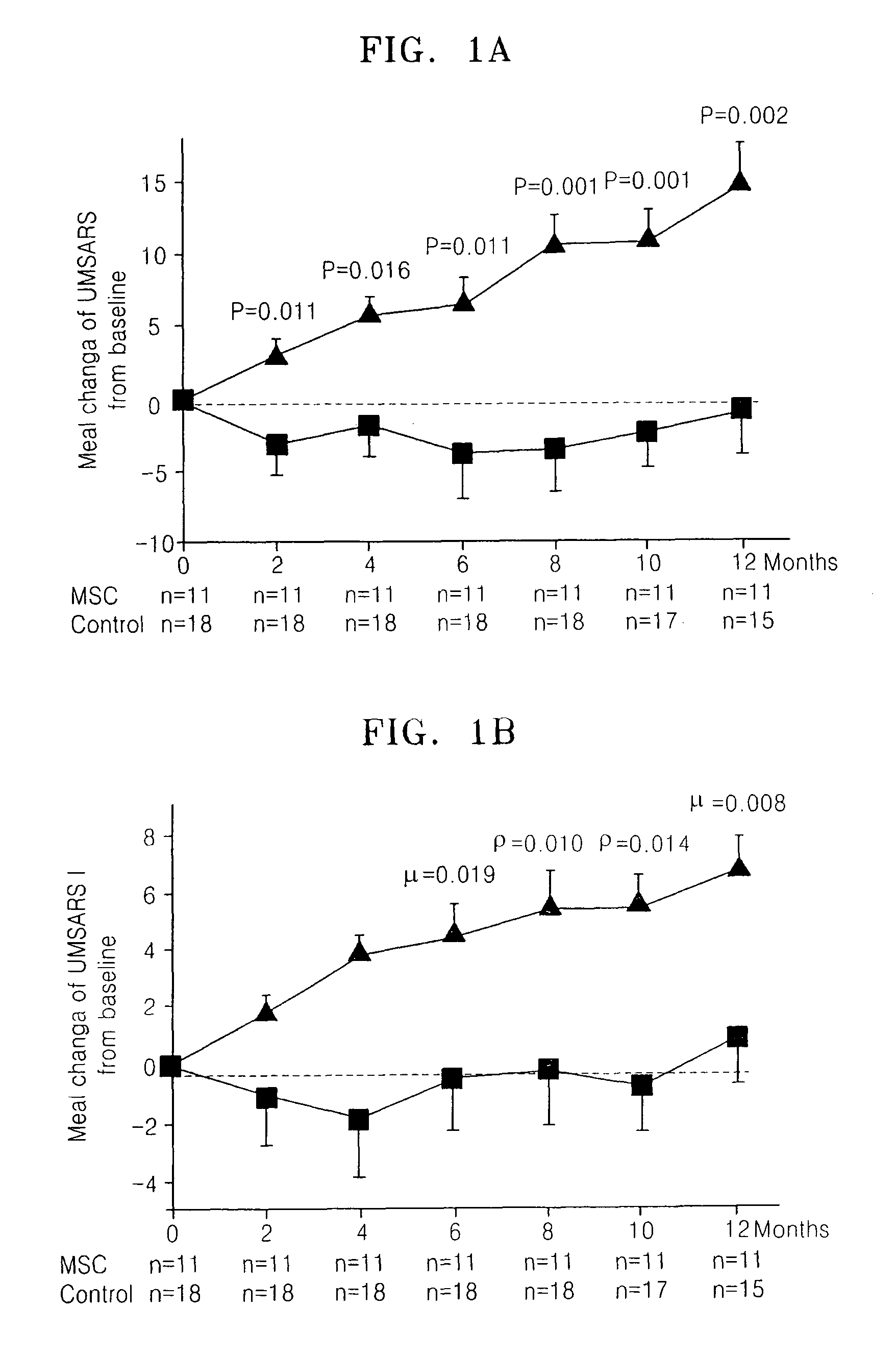
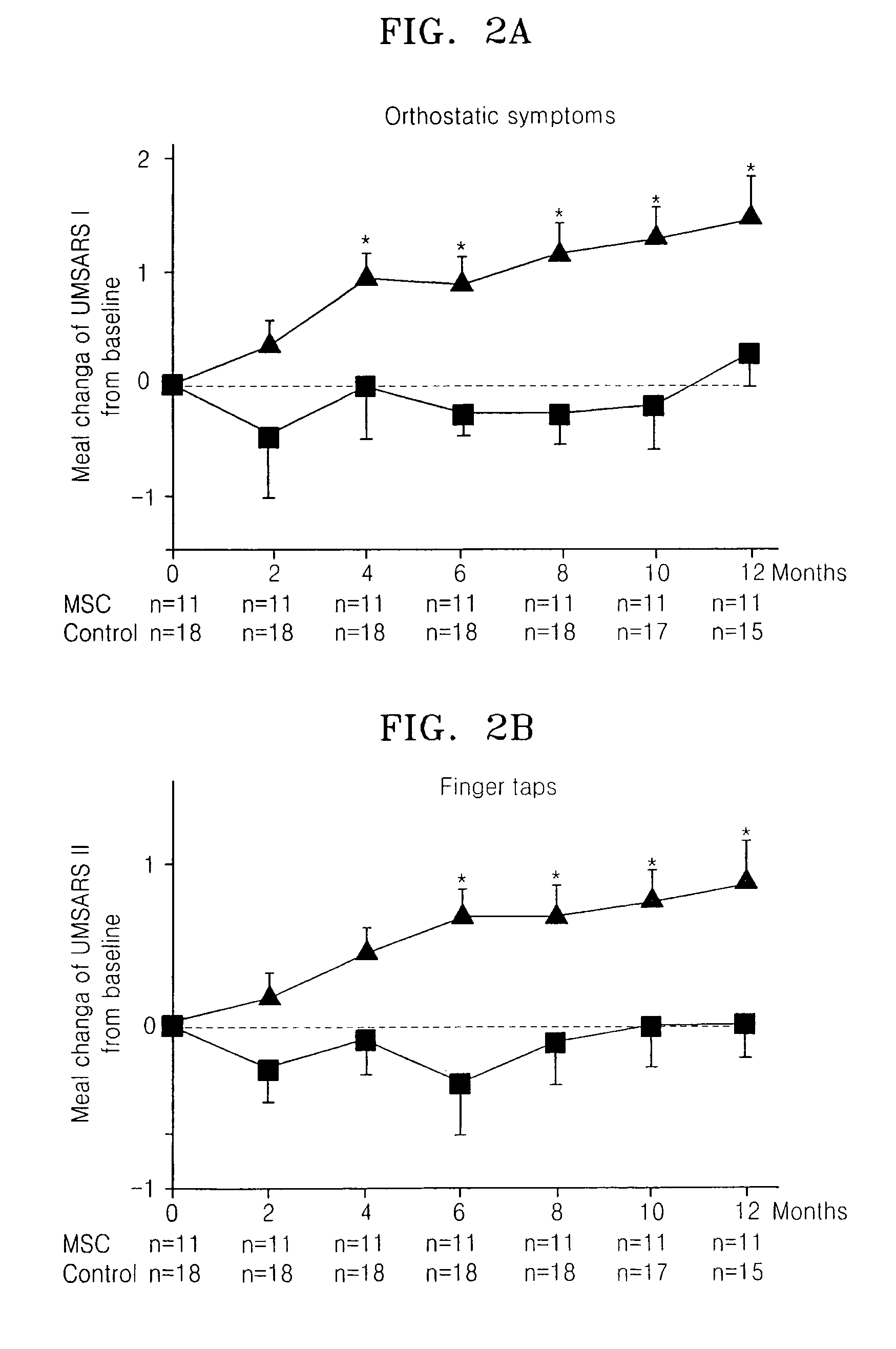
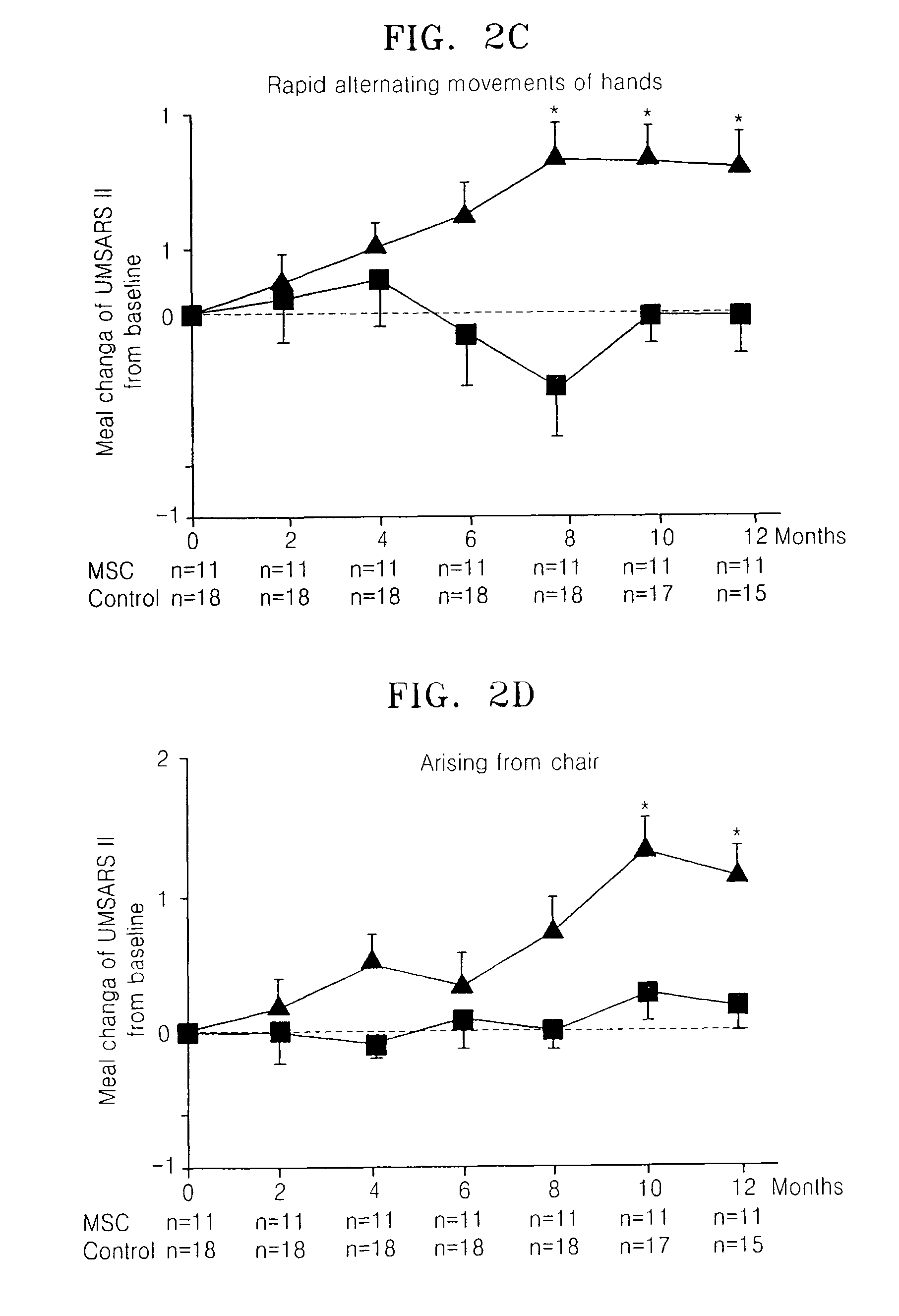
Treating multiple system atrophy with hMSC
The present invention provides a method for treating multiple system atrophy, comprising administering a therapeutically effective amount of mesenchymal stem cells (MSCs) to a human in need thereof. Preferably, the administering is performed by an intra-arterial injection of said MSCs and one or more intravenous injections of said MSCs.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Yeast ectopically expressing abnormally processed proteins and uses therefor
Disclosed are yeast ectopically expressing abnormally processed proteins and methods of screening to identify compounds that modulate the function of such abnormally processed proteins in yeast. Compounds identified by such screens can be used to treat or prevent diseases associated with abnormally processed proteins or protein misfolding. Such diseases include Parkinson's Disease, Parkinson's Disease with accompanying dementia, Lewy body dementia, Alzheimer's disease with Parkinsonism, and multiple system atrophy.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Quinolinone farnesyl transferase inhibitors for the treatment of synucleinopathies and other indications
InactiveUS8232402B2Low toxicityReduce accumulationBiocideNitro compound active ingredientsNervous systemFarnesyl Transferase Inhibitor
Novel quinolinone farnesyl transferase inhibitors are provided. These new compounds are useful in the treatment or prevention of synucleinopathies, such as Parkinson's Disease, Diffuse Lewy Body Disease, multiple system atrophy, and disorders of brain iron concentration including pantothenate kinase-associated neurodegeneration (e.g., PANK1), or other neurodegenerative / neurological diseases. Provided compounds are also useful in the treatment of proliferative diseases such as cancer, and in the treatment of neurological diseases, such as cognitive impairment, depression, and anxiety. The treatment including administering to a subject a therapeutically effective amount of an inventive farnesyl transferase inhibitor compound.
Owner:ASTRAZENECA AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com