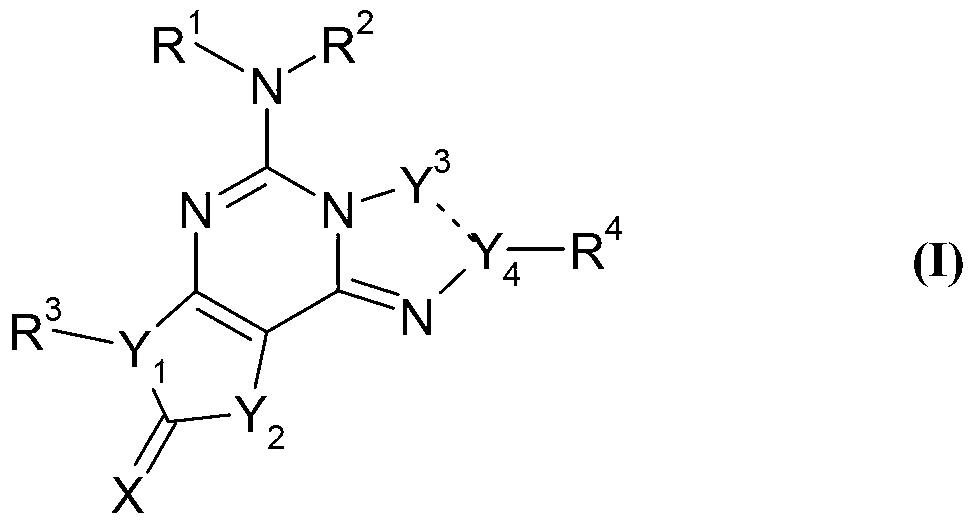
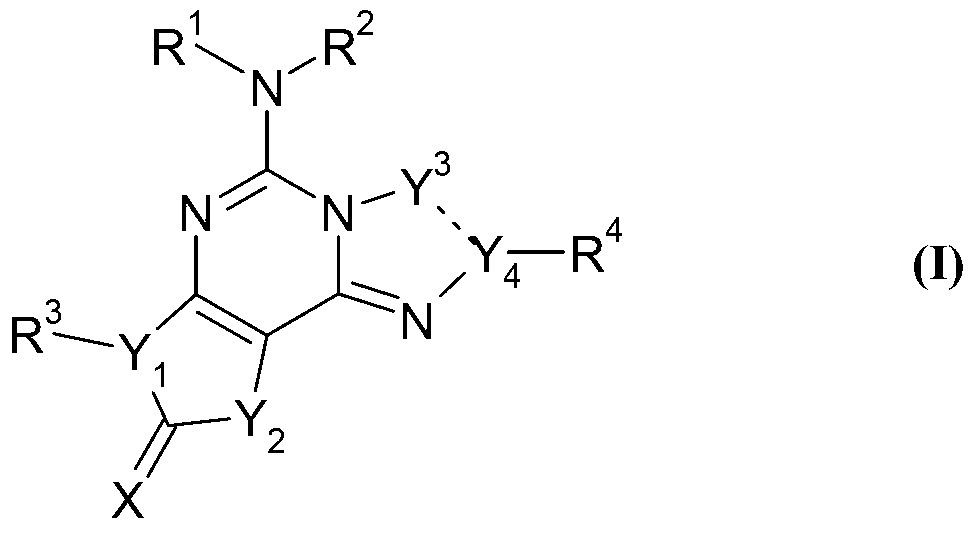
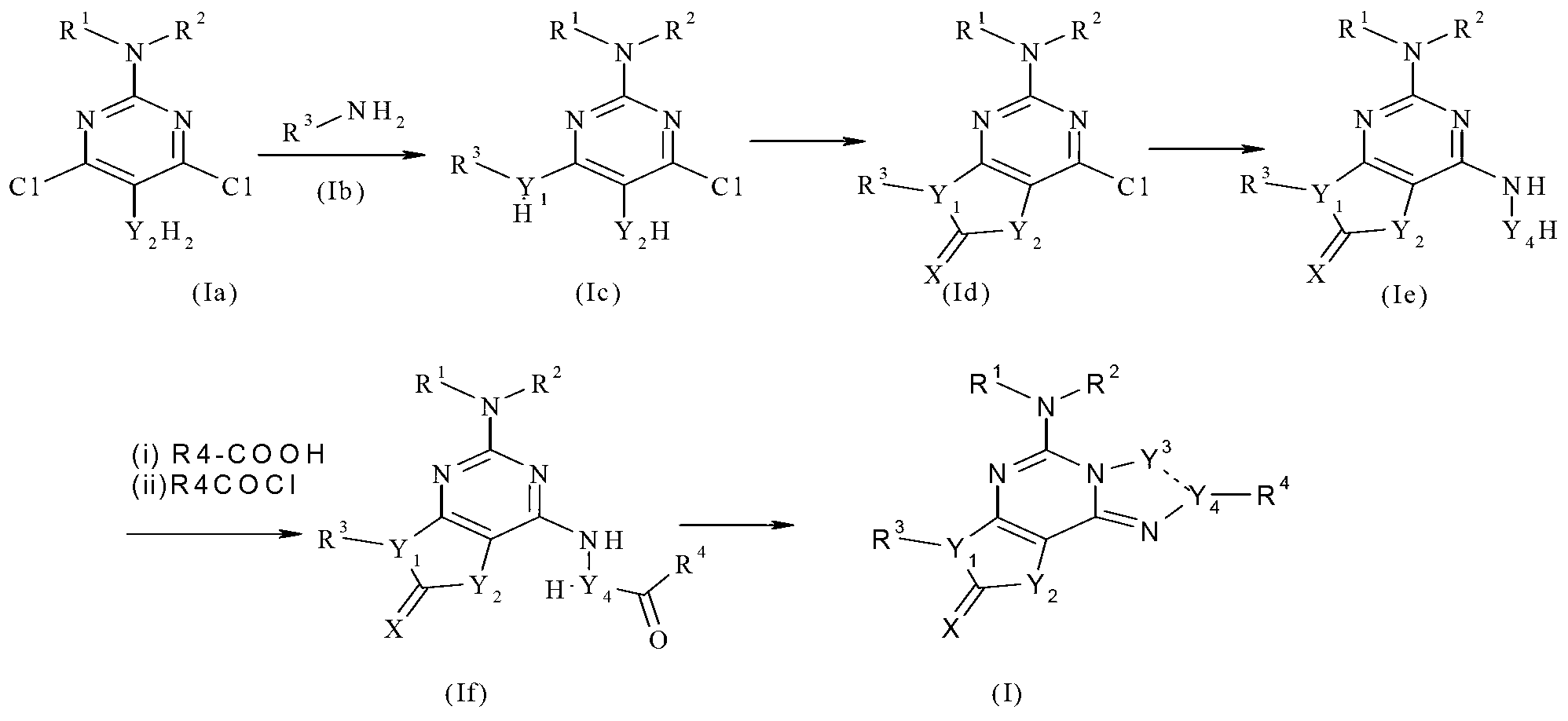
Fused tricyclic compounds as adenosine receptor antagonist
A technology of compounds and solvates, applied in the field of fused tricyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0430] Example A1: 5-amino-8-(2-furyl)-3-[2-[4-[4-(2-methoxyethoxy)phenyl]piperazin-1-yl]ethyl ]-1-Methyl-[1,2,4]triazolo[5,1-f]purin-2-one
[0431]
[0432] Step-1: 2-[(2,5-Diamino-6-chloro-pyrimidin-4-yl)amino]ethanol
[0433] A mixture of 4,6-dichloropyrimidine-2,5-diamine (28g, 156mmol), ethanolamine (18ml, 312mmol) and ethanol (250ml) was heated at 100-110°C for 16 hours. The mixture was cooled and the solvent was removed. Methanol (100 ml) was added to the residue and stirred for 20 minutes. The solid was filtered off to obtain 2-[(2,5-diamino-6-chloro-pyrimidin-4-yl)amino]ethanol (22.0 g, 70%).
[0434] 1 HNMR(400MHz,DMSO d6):δ3.36-3.40(m,2H);3.50-3.54(m,2H);3.88(bs,2H);4.74(t,J=5.6Hz,1H);5.63(bs ,2H);6.51(t,J=5.6Hz,1H)
[0435] Step-2: 2-Amino-6-chloro-9-(2-hydroxyethyl)-7H-purin-8-one
[0436] A mixture of 2-[(2,5-diamino-6-chloro-pyrimidin-4-yl)amino]ethanol (10.0 g, 49.26 mmol) obtained in step 1 in acetonitrile (400 ml) was cooled to 0°C. To the reaction...
Embodiment B1
[0474] Example B1: 5-amino-8-(2-furyl)-3-[2-[4-[4-(2-hydroxyethoxy)phenyl]piperazin-1-yl]ethyl]- 1-Methyl-[1,2,4]triazolo[5,1-f]purin-2-one
[0475]
[0476] To the compound 5-amino-8-(2-furyl)-3-[2-[4-[4-(2-methoxyethoxy)phenyl]piperazin-1-yl] at 0°C Ethyl]-1-methyl-[1,2,4]triazolo[5,1-f]purin-2-one (Example A1) (0.075g, 0.140mmol) in DCM (10ml) The solution was added dropwise to BBr 3 (0.15ml, 0.154mmol), and stirred at 25°C for 20 hours. use sat.NaHCO 3 (25ml) to quench the reaction mixture and extract with DCM (3 x 20ml). The crude product was purified by column chromatography to obtain 5-amino-8-(2-furyl)-3-[2-[4-[4-(2-hydroxyethoxy)phenyl]piperazine as an off-white solid -1-yl]ethyl]-1-methyl-[1,2,4]triazolo[5,1-f]purin-2-one (70 mg, 90%).
[0477] HNMR(400MHz,DMSO d6):δ2.60(bs,4H);2.67(t,J=6Hz,2H);2.95(bs,4H);3.55(s,3H);3.65(q,J=5.2Hz ,2H);3.87(t,J=5.2Hz,2H);3.95(t,J=6.4Hz,2H);4.8(t,J=5.2Hz,1H);6.71-6.72(m,1H);6.78 -6.85(m,4H);7.19(d,J=2.8Hz,1H);7.79(bs,2H);7...
Embodiment C1
[0480] Example C1: 5-amino-1-(cyclopropylmethyl)-3-[2-[4-(4-ethoxyphenyl)piperazin-1-yl]ethyl]-8-(2 -furyl)-[1,2,4]triazolo[5,1-f]purin-2-one
[0481]
[0482] Step-1: 2-Amino-6-chloro-7-(cyclopropylmethyl)-9-(2-hydroxyethyl)purin-8-one (the procedure is the same as Step-3 in Example A1)
[0483] 1 HNMR(400MHz,DMSO d6):δ0.35-0.39(m,2H);0.41-0.49(m,2H);1.16-1.23(m,1H),3.63-3.67(m,2H);3.77-3.81( m,4H);4.87(t,J=5.2Hz,1H);6.71(bs,2H)
[0484] Step-2: 2-Amino-7-(cyclopropylmethyl)-6-hydrazino-9-(2-hydroxyethyl)purin-8-one (the procedure is the same as Step-4 in Example A1)
[0485] 1 HNMR(400MHz,DMSO d6):δ0.28-0.30(m,2H);0.35-0.37(m,2H);1.00-1.04(m,1H),3.57-3.66(m,2H);3.72-3.79( m,4H);4.33(bs,2H);4.88(t,J=5.6Hz,1H);6.00(bs,2H);7.52(bs,1H).
[0486] Step-3: N'-[2-amino-7-(cyclopropylmethyl)-9-(2-hydroxyethyl)-8-oxo-purin-6-yl]furan-2-carbohydrazide (step is identical with step-5 in embodiment A1)
[0487] The crude product was used in the next step.
[0488] Step-4: 5-Am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com