Method for enriching or detecting astrocyte-derived exosomes from biological fluids
A technology of astrocytes and biological fluids, applied in the field of biomedicine, can solve the problems of routine application of traumatic operations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
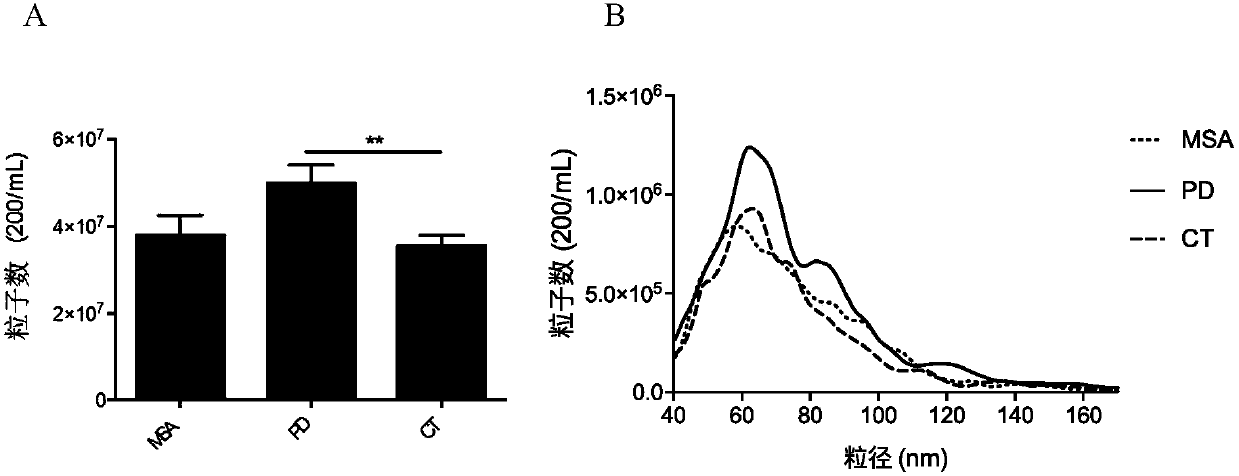
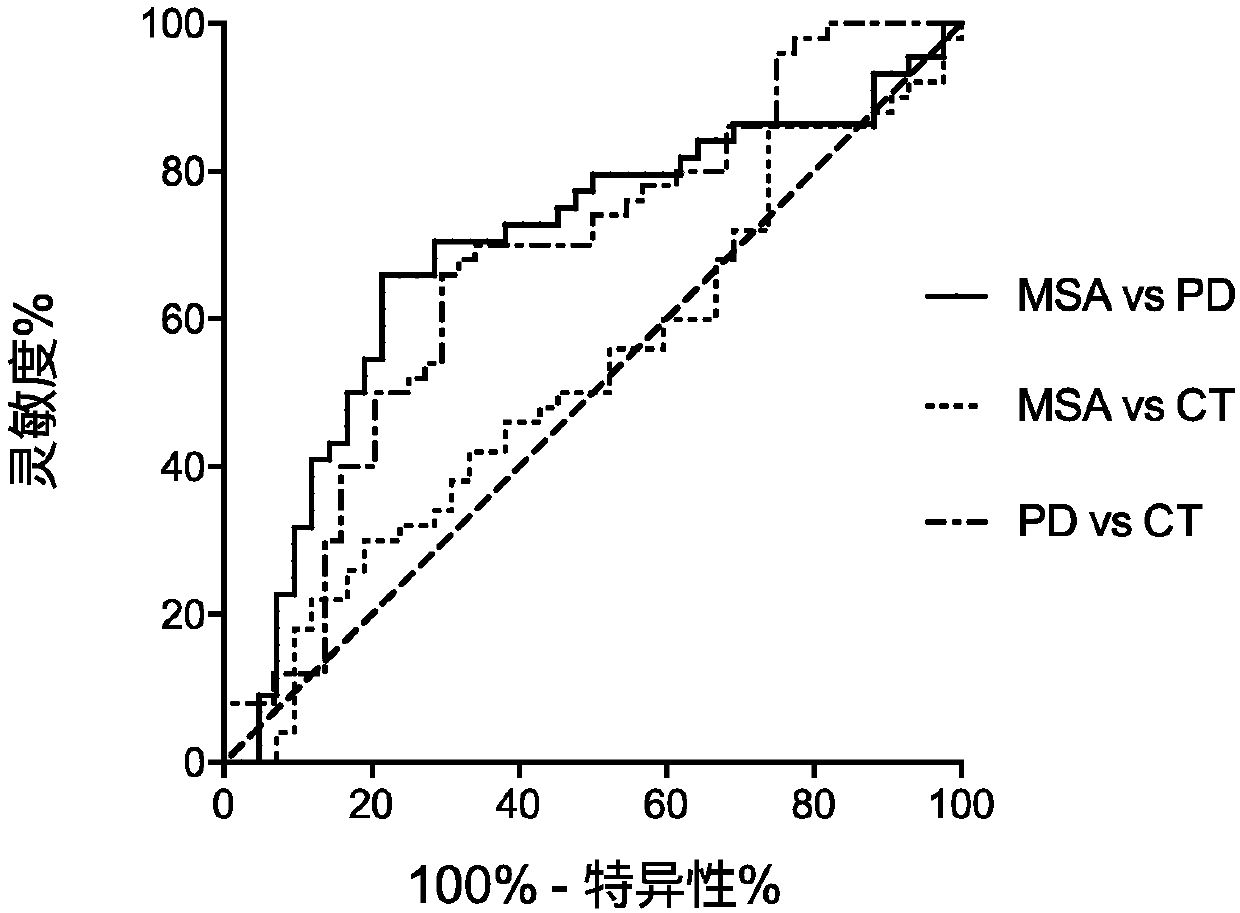
[0094] According to a typical embodiment of the present invention, the step (b) includes: (b) binding the astrocyte-derived exosomes in the biological fluid to a solid phase through an immune complex; (c) separating the solid phase binding exosomes and (d) determining the level of astrocyte-derived biomarkers in the solid-phase-bound exosomes isolated in step (c), and, compared with those from subjects without neurological disease Compared with the control level of , the difference in the level of biomarkers from astrocyte-derived exosomes in the subjects significantly indicates that the subjects suffer from neurological diseases.
[0095] In another typical embodiment of the present invention, when the biomarker is astrocyte-derived exosomes themselves, the anti-GLT1 antibody is a labeled antibody, and step (b) includes: directly detecting the astrocytes in the biological fluid by means of the label Stemocyte-derived exosomes; the labeling of anti-GLT1 antibodies includes but...
Embodiment 1
[0099] Exosome Isolation
[0100] Exosomes were isolated from mouse or human plasma following a protocol modified from Tauro et al (Methods 56:293-304, 2012) using antibody-coated superparamagnetic microbeads.
[0101] Brief description: 10 μg of anti-GLT1 antibody (ab178401, Abcam, Cambridge, MA, USA), or normal mouse IgG (Santa Cruz Biotechnology, Dallas, TX, USA) (as a negative control) was coated on a group (1 mg) of M -270 epoxy beads using an antibody conjugation kit (Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. After rapid thawing (within 2 minutes) at 37°C, plasma samples (>300 μL) were centrifuged at 2,000ⅹg for 15 minutes, followed by 12,000ⅹg for 30 minutes, and then washed with phosphate-buffered saline (PBS) (pH 7.4 ) to dilute the supernatant 1:3. One set of antibody-coated beads and 900 [mu]L diluted plasma were incubated for approximately 24 hours at 4[deg.] C. with gentle rotation. The beads were then washed four t...
Embodiment 2
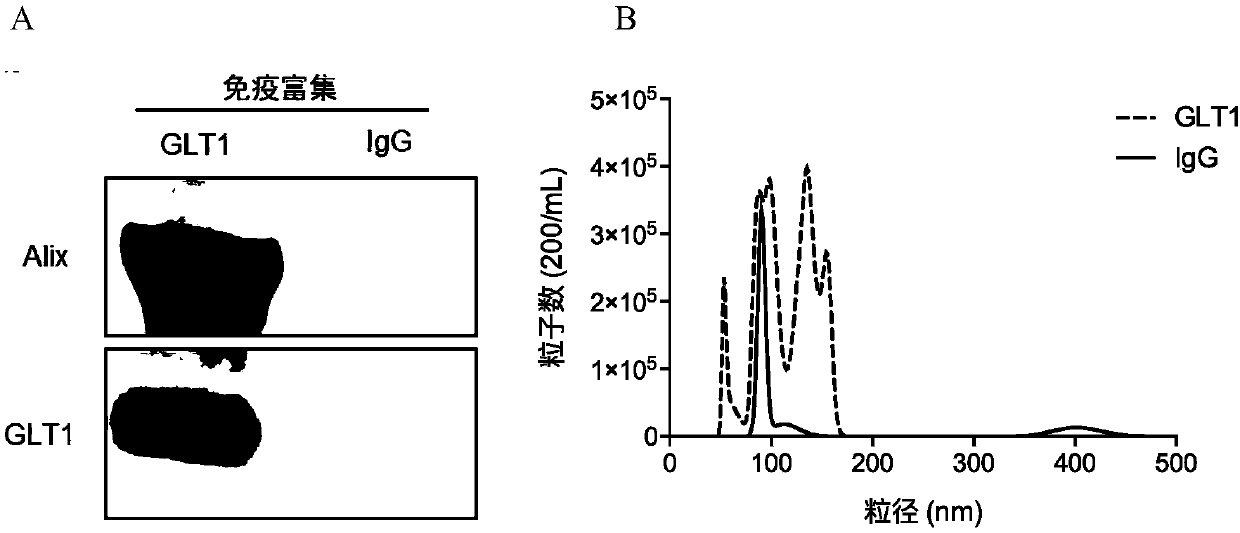
[0105] Characterization of anti-GLT1-trapped exosomes from human plasma
[0106] Luminex assay: α-syn, oligomer a-syn, PS129, tau, Aβ protein concentration.
[0107] Western Blot Analysis: Western blots were performed following standard protocols. Exosome samples (~10 μg protein) were lysed with Laemmli sample buffer and separated on 1D SDS-PAGE gels before transferring to polyvinylidene fluoride membranes. Proteins on the above membranes were detected with the aid of the following primary antibodies: mouse anti-human GLT1 (Abcam, 1:500) and mouse anti-human Alix (Cat#ABC40, Millipore, Billerica, MA, USA; 1:1000).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com