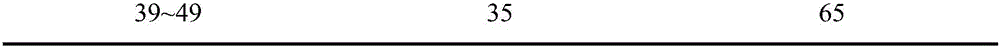A kind of method for detecting glycosides
A detection method and drug technology, applied in the field of pharmaceuticals, can solve the problems of no preparation quality detection method and inability to effectively guarantee product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0323] Example 1: Determination of Monoester Alkaloid Content in Fugan Granules
[0324] 1) Preparation of granules: Take 6 kg of aconite and 1 kg of licorice, add water to decoct twice, add 8 times the amount of water for the first time, decoct for 2 hours, add 6 times the amount of water for the second time, decoct for 1 hour, combine twice The decoction is filtered, concentrated, dried under reduced pressure, crushed, an appropriate amount of dextrin is added, mixed evenly, granulated by wet method, dried, and granulated to obtain granules of the medicine with sweetener.
[0325] 2) inspection
[0326] Get sample, check according to quality standard described in the present invention, test result sees the following table:
[0327] Table 38 Test results
[0328]
[0329]
Embodiment 2
[0330] Embodiment 2: Determination of monoester type alkaloid content of Fugan Granules
[0331] 1) Preparation of granules: Take 18kg of aconite and 9kg of licorice, add water to decoct twice, add 10 times the amount of water for the first time, decoct for 1.5 hours, add 8 times the amount of water for the second time, decoct for 1 hour, combine twice The decoction is filtered, concentrated, dried under reduced pressure, crushed, an appropriate amount of dextrin is added, mixed evenly, granulated by wet method, dried, and granulated to obtain granules of the medicine with sweetener.
[0332] 2) inspection
[0333] Get sample, check according to quality standard described in the present invention, test result sees the following table:
[0334] Table 39 Test results
[0335]
[0336]
Embodiment 3
[0337] Embodiment 3: Determination of monoester type alkaloid content of Fugan Granules
[0338] 1) Preparation of granules: Take 10kg of aconite and 5kg of licorice, add water to decoct three times, add 8 times the amount of water for the first time, decoct for 1.5 hours, add 8 times the amount of water for the second time, decoct for 1 hour, and add 6 times the amount of water, decocted for 1 hour, combined three decoctions, filtered, concentrated, dried under reduced pressure, pulverized, added an appropriate amount of dextrin, mixed evenly, wet granulated, dried, and granulated to obtain granules of Fugan drug .
[0339] 2) inspection
[0340] Get sample, check according to quality standard described in the present invention, test result sees the following table:
[0341] Table 40 Test results
[0342]
[0343]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com