Magnetic acrylamide hydrogel and preparation method thereof
A technology of acrylamide and hydrogel, applied in the field of magnetic acrylamide hydrogel and its preparation, can solve the problems such as the unseen reports of particle method, and achieve the improvement of biocompatibility, clarity, good size and good size. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Dissolve 0.7282g N-isopropylacrylamide NtBAM, 0.0467g acrylic acid AAc, 0.0212g N,N'-methylenebisacrylamide and 0.0402g sodium lauryl sulfate in 90g deionized water, and Bubble N at room temperature 2 Deoxygenation, magnetic stirring for 120min;
[0037] (2) The reaction temperature rises to 60°C, under N 2 Insulate the above solution under protection for 40min;
[0038] (3) then preparation mass concentration is the ammonium persulfate solution of 0.6%, gets 9.9987g and adds in the above-mentioned solution, keeps N 2 atmosphere, continue to react for 3h;
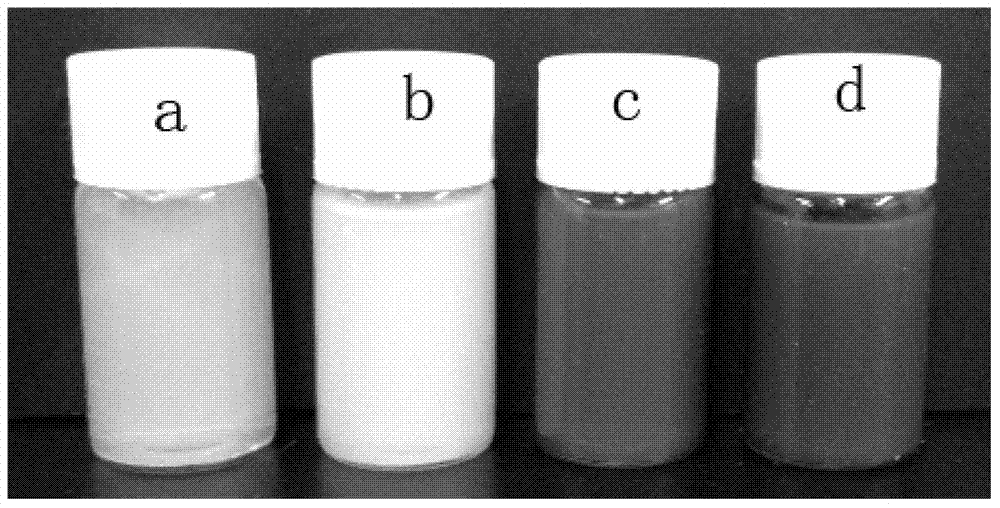
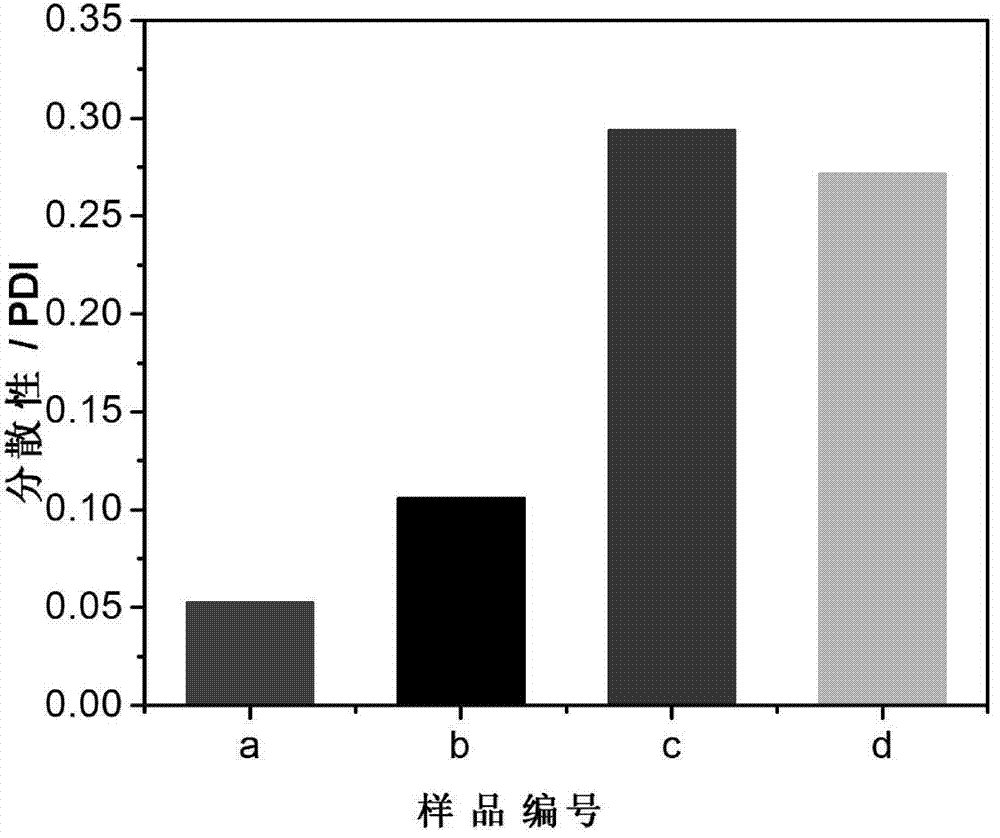
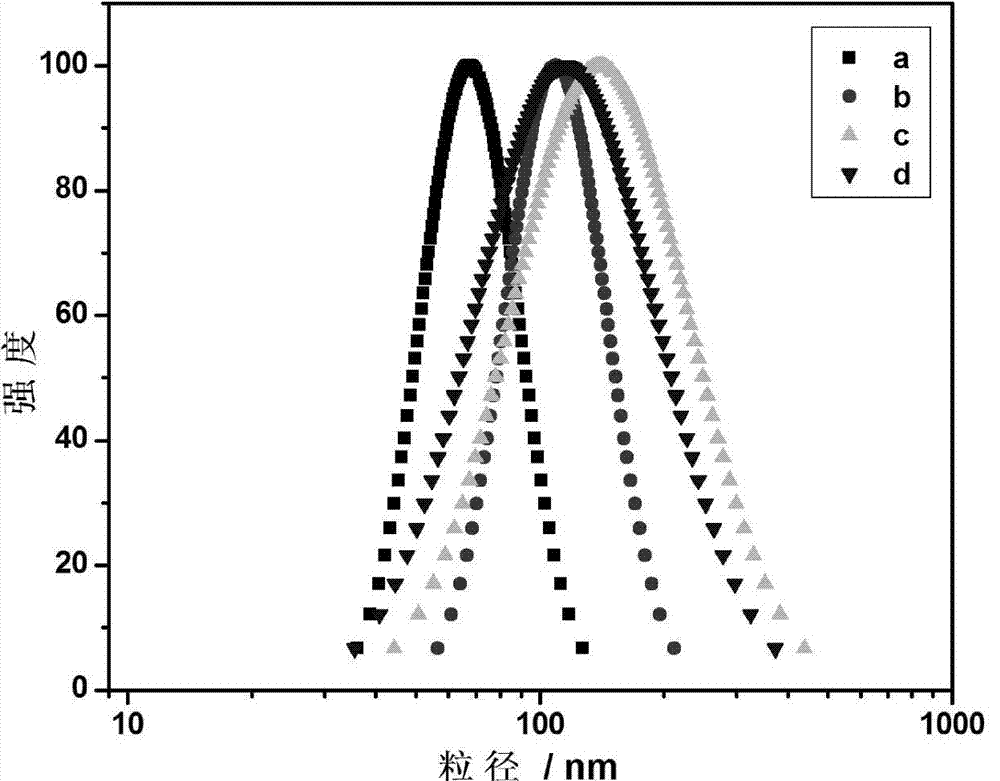
[0039] (4) Soak the obtained reactant in deionized water and dialyze for 5 days, change the water twice a day, remove the residual reaction raw materials and electrolyte in the reaction system, and use a dialysis bag with a molecular weight cut-off of 8000-14000 to obtain the copolymerization of NtBAM and AAc Hydrogels, numbered a, such as figure 1 shown.
Embodiment 2
[0041] (1) Take 0.7259g N-isopropylacrylamide NtBAM, 0.2740g acrylic acid AAc, 0.0302g N,N'-methylenebisacrylamide and 0.0205g sodium lauryl sulfate dissolved in 90g deionized water, and Bubble N at room temperature 2 Deoxygenation, magnetic stirring for 120min;
[0042] (2) The reaction temperature rises to 60°C, under N 2 Insulate the above solution under protection for 40min;
[0043] (3) then prepare the ammonium persulfate solution that mass concentration is 0.6%, get 10.0087g and add in the above-mentioned solution, keep N 2 atmosphere, continue to react for 3h;
[0044] (4) Soak the obtained reactant in deionized water and dialyze for 5 days, change the water twice a day, remove the residual reaction raw materials and electrolyte in the reaction system, and use a dialysis bag with a molecular weight cut-off of 8000-14000 to obtain the copolymerization of NtBAM and AAc Hydrogel, numbered b, such as figure 1 shown.
Embodiment 3
[0046] (1) Take 50mL of the product in Example 1, use NaOH to adjust the pH to 6.42, and 0.1391g of FeSO 4 ·7H 2 O mixed, at room temperature, N 2 Stir in the atmosphere overnight, then use the above-mentioned dialysis bag for dialysis for 2 hours;
[0047] (2) Continue at room temperature, N 2 After stirring in the atmosphere for 2h, add 0.054gNaNO 2 ;
[0048] (3) Keep stirring for 10 minutes, add 5 mL of ammonia water using a syringe, and continue to react for 3 hours;
[0049] (4) The obtained reactant was soaked in deionized water and dialyzed for 3 days, and the water was changed twice a day, using the above-mentioned dialysis bag. The in situ synthesized magnetic hydrogel is obtained, numbered c, such as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com