Release way and preparation method of novel compound sleeping controlled release preparation
A technology for controlled release preparations and compound recipes, which is applied in the field of pharmaceutical preparations and can solve problems such as slow onset of action and slow drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
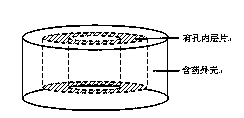
[0031] The controlled-release tablet of this embodiment is as figure 1 As shown, it includes an inner tablet and a drug-containing shell, and the inner tablet is a porous tablet with a slow-release layer.
[0032] The composition and content of the controlled-release tablets are as follows: based on 1000 tablets, each tablet weighs 450 mg.
[0033] Drug-containing shell:
[0034]
[0035] Perforated inner sheet:
[0036]
[0037] Preparation:
[0038] 1. Weigh the raw and auxiliary materials of the inner layer tablet according to the prescription, and mix them thoroughly for later use; select two types of punches, and the diameter of No. 1 punch is smaller than that of No. Ⅱ;
[0039] 2. Use a rotary tablet press, install punch Ⅰ, adjust the filling depth and tablet pressing force of the tablet press, and press the inner tablet. The tablet weight is 150 mg per tablet, and the hardness of the inner tablet is 80 N;
[0040] 3. Use a rotary tablet press, install punch I...
Embodiment 2
[0044] The controlled-release tablet of this embodiment is as figure 1 As shown, it includes an inner tablet and a drug-containing shell, and the inner tablet is a porous tablet with a slow-release layer.
[0045]The composition and content of the controlled-release tablets are as follows: based on 1000 tablets, each tablet weighs 450 mg.
[0046] Drug-containing shell:
[0047]
[0048] Perforated inner sheet:
[0049]
[0050] Preparation:
[0051] 1. Weigh the raw and auxiliary materials of the inner layer tablet according to the prescription, and mix them thoroughly for later use; select two types of punches, and the diameter of No. 1 punch is smaller than that of No. 2;
[0052] 2. Use a rotary tablet press, install punch Ⅰ, adjust the filling depth and tablet pressing force of the tablet press, and press the inner tablet. The tablet weight is 150 mg per tablet, and the hardness of the inner tablet is 80 N;
[0053] 3. Use a rotary tablet press, install punch II...
Embodiment 3
[0057] The controlled-release tablet of this embodiment is as figure 1 As shown, it includes an inner tablet and a drug-containing shell, and the inner tablet is a porous tablet with a slow-release layer.
[0058] The composition and content of the controlled-release tablets are as follows: based on 1000 tablets, each tablet weighs 450 mg.
[0059] Drug-containing shell:
[0060]
[0061] Perforated inner sheet:
[0062]
[0063] Preparation:
[0064] 1. Weigh the raw and auxiliary materials of the inner layer tablet according to the prescription, and mix them thoroughly for later use; select two types of punches, and the diameter of No. 1 punch is smaller than that of No. Ⅱ;
[0065] 2. Use a rotary tablet press, install punch Ⅰ, adjust the filling depth and tablet pressing force of the tablet press, and press the inner tablet. The tablet weight is 150 mg per tablet, and the hardness of the inner tablet is 80 N;
[0066] 3. Use a rotary tablet press, install punch I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com