Amide derivatives of aniline-related compound and composition of amide derivatives of aniline-related compound
A compound and solvate technology, applied in the field of amide derivatives, can solve the problems of limited bioavailability and low water solubility of aniline-related compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] Preparation of Amide Derivatives Disclosed herein.
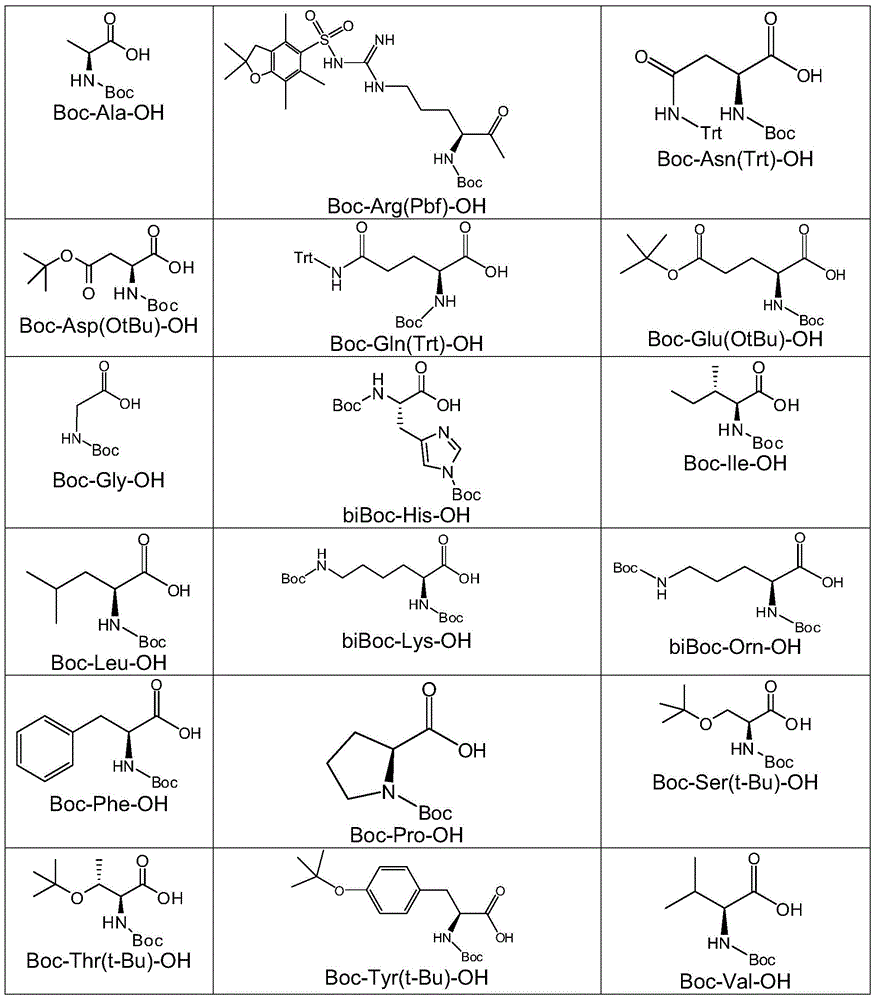
[0110] Amide derivatives of aniline-related compounds can be prepared by conventional organic synthesis. For example, amide derivatives can be prepared by reacting an aniline related compound with a suitable acid, where the other reactive group of the acid is protected by a protecting group (e.g. amino group via tert-butoxycarbonyl (BOC), trityl (Trt) or 2,2,4,6,7-pentamethyl-2,3-dihydrobenzofuran-5-ylsulfonyl (Pbf) protected; hydroxyl by tert-butoxy (tBu); carboxyl by tert-butyl carboxylate (OtBU) protection) to avoid undesired reactions. In certain embodiments, a coupling agent such as HBTU (O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate) and It is carried out under the condition of base (such as DIEA (N,N-diisopropylethylamine)).
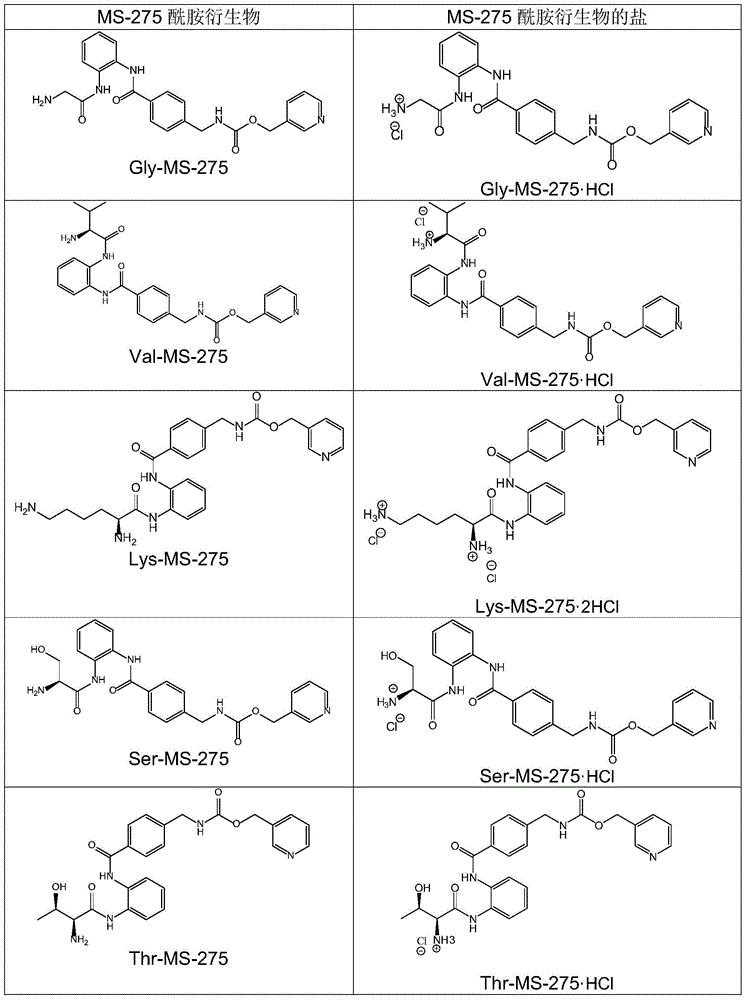
[0111] The prepared amide derivatives, if provided with protecting groups ("protected amide derivatives"), can be further converted into unprotected amide deri...
Embodiment 1
[0130] Example 1: Preparation of amide derivatives of aniline-related compounds disclosed herein.
[0131] Several amide derivatives of several aniline-related compounds were prepared from the reaction of the corresponding aniline-related compounds and acids in DMF and in the presence of HBTU and DIEA. Other active groups on the acid that should not participate in the reaction have been protected (such as the amino group through tert-butoxycarbonyl (BOC), trityl (TrtRT protection) or 2,2,4,6,7-pentamethyl -2,3-dihydrobenzofuran-5-ylsulfonyl (Pbf) protected; hydroxyl protected by tert-butoxy (tBu); carboxyl protected by tert-butyl carboxylate (OtBU)).
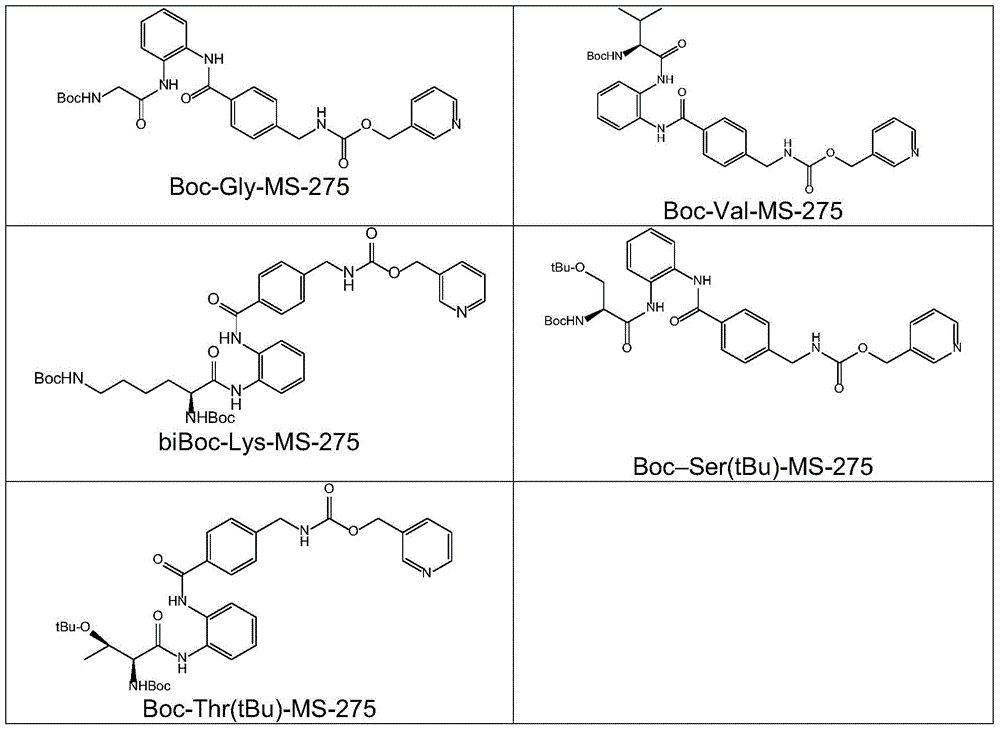
[0132] I. Preparation of MS-275 Amide Derivatives.
[0133] To a solution of the acid in DMF (20 mL) was added HBTU and DIEA. The reaction mixture was stirred at 10°C for 10 minutes, and MS-275 was added to the reaction solution. After the reaction mixture was stirred at room temperature for 12 hours, 50 ml of water was added...
Embodiment 2
[0218] Example 2. Solubility of several amide derivatives.
[0219] Solubility (mg / ml) of a compound in water was measured by adding the compound to a known amount (eg, 1 mL) of water until saturation, and measuring the amount of the compound added (Table 9).
[0220] Table 9. Solubility of several amide derivatives.
[0221] Structural formula PR
[0222] Structural formula PR-4
[0223] Structural formula PR-3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com