Application of Babaodan in preparation of drugs for treating pulmonary fibrosis
A pulmonary fibrosis and idiopathic technology, applied in the field of application of Babao Dan in the preparation of drugs for the treatment of idiopathic pulmonary fibrosis, can solve the problems of application limitations, large side effects, inconsistent curative effects, etc., and achieve therapeutic efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
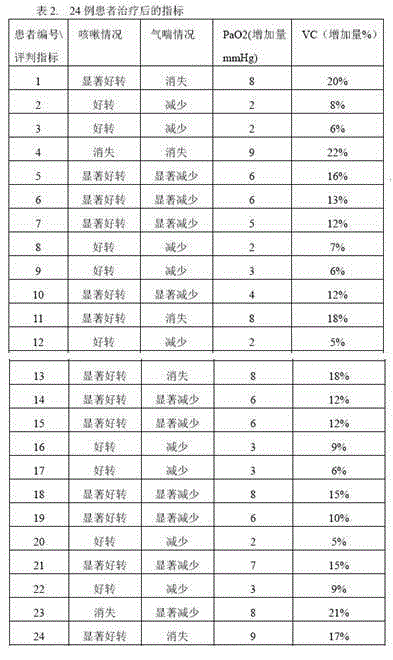
[0019] Take commercially available Babaodan capsules (Guoyao Zhunzi Z10940006, produced by Xiamen Traditional Chinese Medicine Factory Co., Ltd.), each capsule contains 0.25-0.3g of Babaodan, and it is used in the preparation of drugs for the treatment of idiopathic pulmonary fibrosis. 3 times a day, 2 capsules each time.
Embodiment 2
[0021] Take commercially available Babaodan lozenges (Z10940005, produced by Xiamen Traditional Chinese Medicine Factory Co., Ltd.) and pulverize them, add conventional excipients to make tablets, and each tablet contains 0.25-0.3g of Babaodan, which is used to prepare the treatment of idiopathic hair loss. In the drug of chronic pulmonary fibrosis, when the patient takes it, 2 times a day, 2 tablets each time.
Embodiment 3
[0023] Take commercially available Babaodan lozenges (Z10940005, produced by Xiamen Traditional Chinese Medicine Factory Co., Ltd.) and pulverize them, add conventional excipients to make granules, and each bag of granules contains 0.3-0.5 g of Babaodan, which is used for the preparation of therapeutic drugs. In the drug for paroxysmal pulmonary fibrosis, when the patient takes it, 2 sachets each time, 2 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com