Application of Turrapubin D in preparation of liver-protecting drug
A technology of turrapubind, 1. turrapubind is applied in the application field of Turrapubin D in the preparation of hepatoprotective drugs, and can solve problems such as no reports of compound activity yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
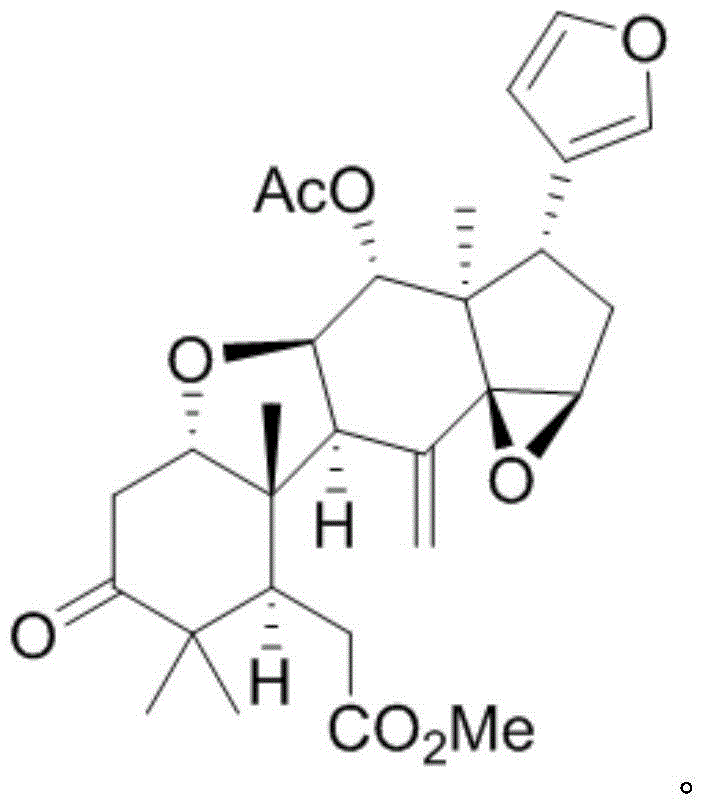
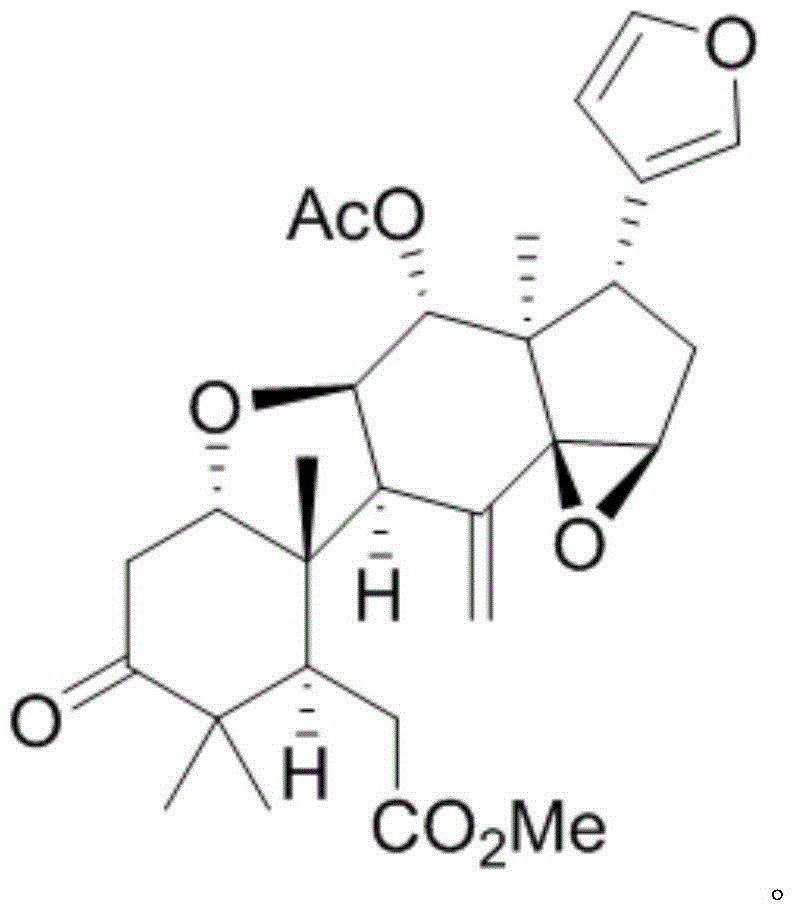
[0009] Example 1: Isolation, preparation and structure confirmation of TurrapubinD
[0010] The preparation method of TurrapubinD is the same as that reported in the literature (Bioactive Limonoid and Triterpenoid Constituents of Turraea pubescens, J. Nat. Prod., 2013, 76, 1166-1174).
[0011] Structural confirmation: According to HRESI-MS, the molecular formula is C 29 h 36 o 8 , with an unsaturation of 12. H NMR spectrum data δ H (ppm, DMSO-d 6 , 500MHz): H-1 (4.12, t, J=5.7), H-2 (2.85, dd, J=14.9, 5.7), H-2 (2.70, dd, J=14.9, 5.7), H-5 (2.63, dd, J=7.5, 3.6), H-6 (2.49, m), H-9 (3.38, d, J=8.7), H-11 (4.35, t, J=8.7), H-12 (5.47, d, J=8.7), H-15 (3.87, s), H-16 (1.80, m), H-16 (2.24, dd, J=14.0, 7.4), H-17 (2.99, dd , J=10.8, 7.4), H-18 (0.80, s), H-19 (1.06, s), H-21 (7.12, s), H-22 (6.16, s), H-23 (7.32, s), H-28 (0.96, s), H-29 (1.11, s), H-30 (5.30, s), 7-OMe (3.71, s), 12-OAc (1.89, s); NMR Carbon spectrum data δ C (ppm, DMSO-d 6 , 125Hz): 82.6 (CH, 1-C), 4...
Embodiment 2
[0013] Embodiment 2: Pharmacological action test of Turrapubin D
[0014] 1. Materials and Instruments
[0015] HL7702 liver cell line was provided by Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. TurrapubinD is self-made, and the HPLC normalized purity is greater than 98%. High-glucose DMEM medium, high-glucose DMEM / F12=1:1 medium, and fetal bovine serum were all purchased from HycLone. EDTA was purchased from Shanghai Reagent No. 1 Factory. Trypan Blue was purchased from Beijing Chemical Plant. Sugar-free DMEM medium was purchased from Gibco. Trypsin, MTT, and DMSO were purchased from Amresco. LDH release assay kit was purchased from GENMDE. ALT biochemical detection kits, AST biochemical detection kits, and LDH biochemical detection kits were purchased from Beckman Reagent Co., Ltd., USA. Western and IP cell lysates, PMSF, BCA protein concentration determination kits, and MDA detection kits were all purchased from Beyontien Biotechnology Res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com