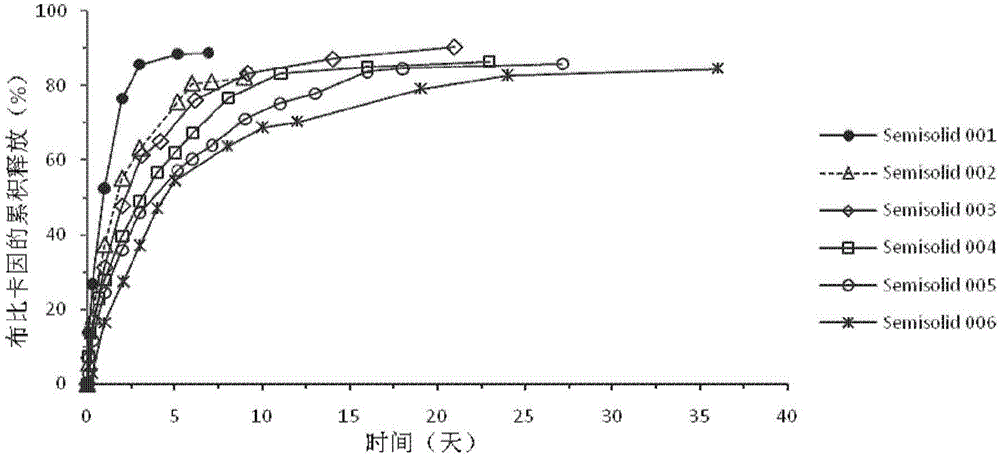
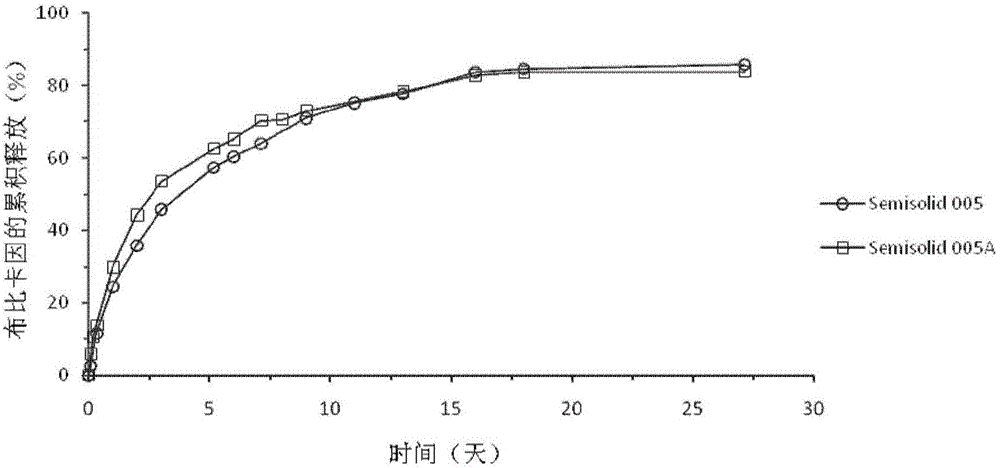
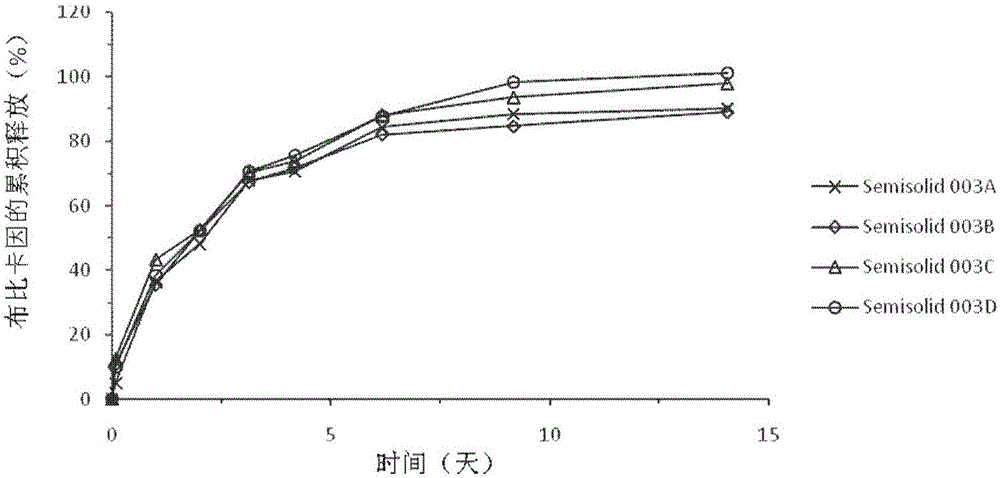
Injectable long-acting local anesthetic semi-solid formulations and its compostions
A semi-solid, active ingredient technology, applied in the field of injectable long-acting local anesthetic semi-solid preparations and their components, can solve the problems of unsatisfactory pain relief, expensive, cumbersome and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0098] Example description
[0099] 1. Preparation of pharmaceutical composition
[0100] Semi-solid local anesthetic pharmaceutical compositions are prepared by adding the local anesthetic, semi-solid lipid, and modified excipients to a glass vessel and heating to approximately 60°C to 95°C, depending on the local anesthetic and carrier composition used The properties of semi-solid lipids and modified excipients are completely melted into a solution, and the active drug is completely dissolved in the drug release carrier to form a transparent solution by constant stirring. After a homogeneous and uniform pharmaceutical composition has been achieved, the semisolid formulation of the local anesthetic may be allowed to cool naturally to ambient temperature.
[0101] A.60wt% S378: 40wt% Tetracaine / Lidocaine (1:2)
[0102] After heating to 60°C, all the compositions were completely melted, and after the two local anesthetics were dissolved, all the compositions formed a transpar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity value | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com