Solid dispersions of selective progesterone receptor modulators
A solid dispersion, active ingredient technology, applied in the direction of organic active ingredients, diseases, pill delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
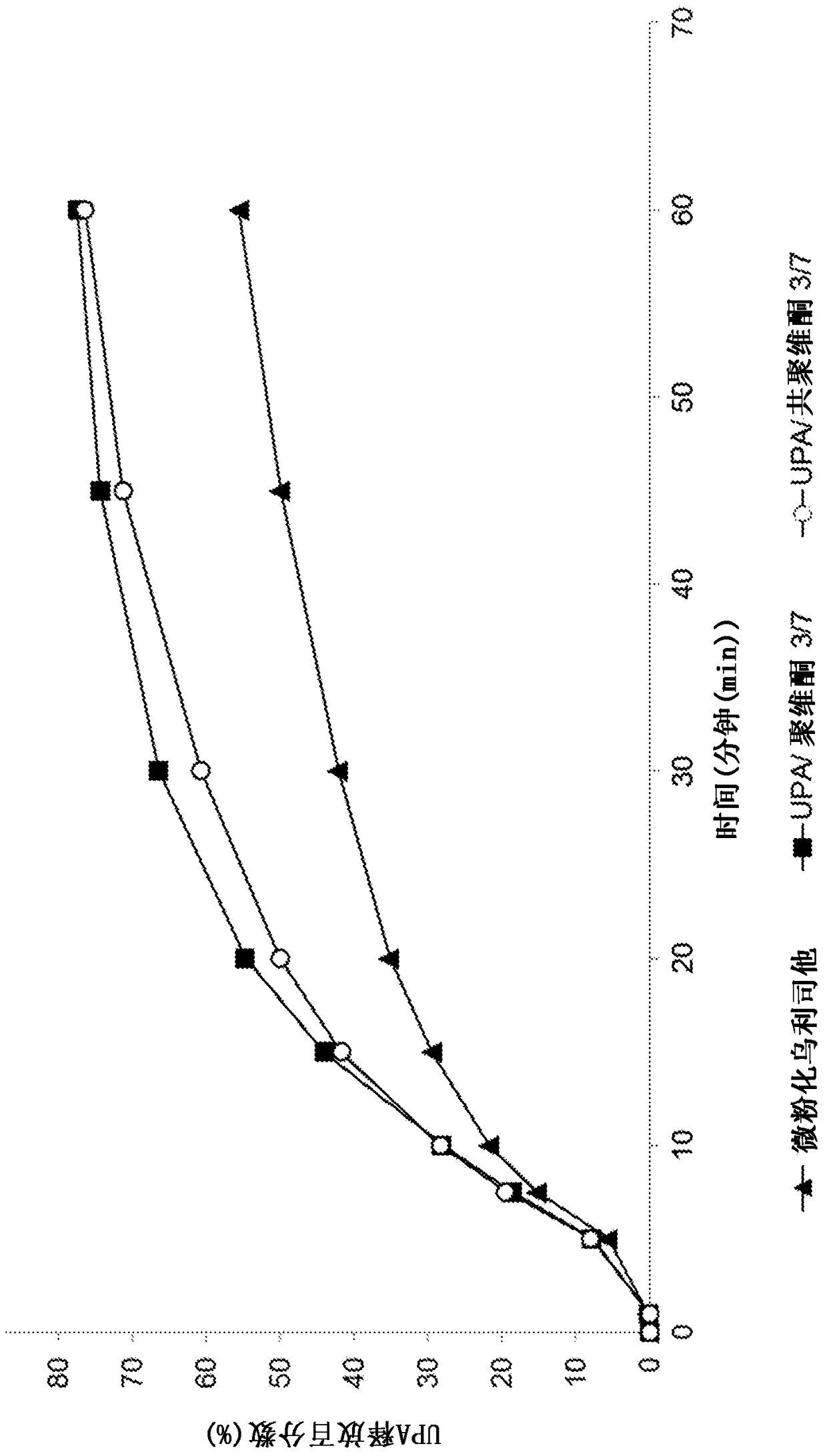
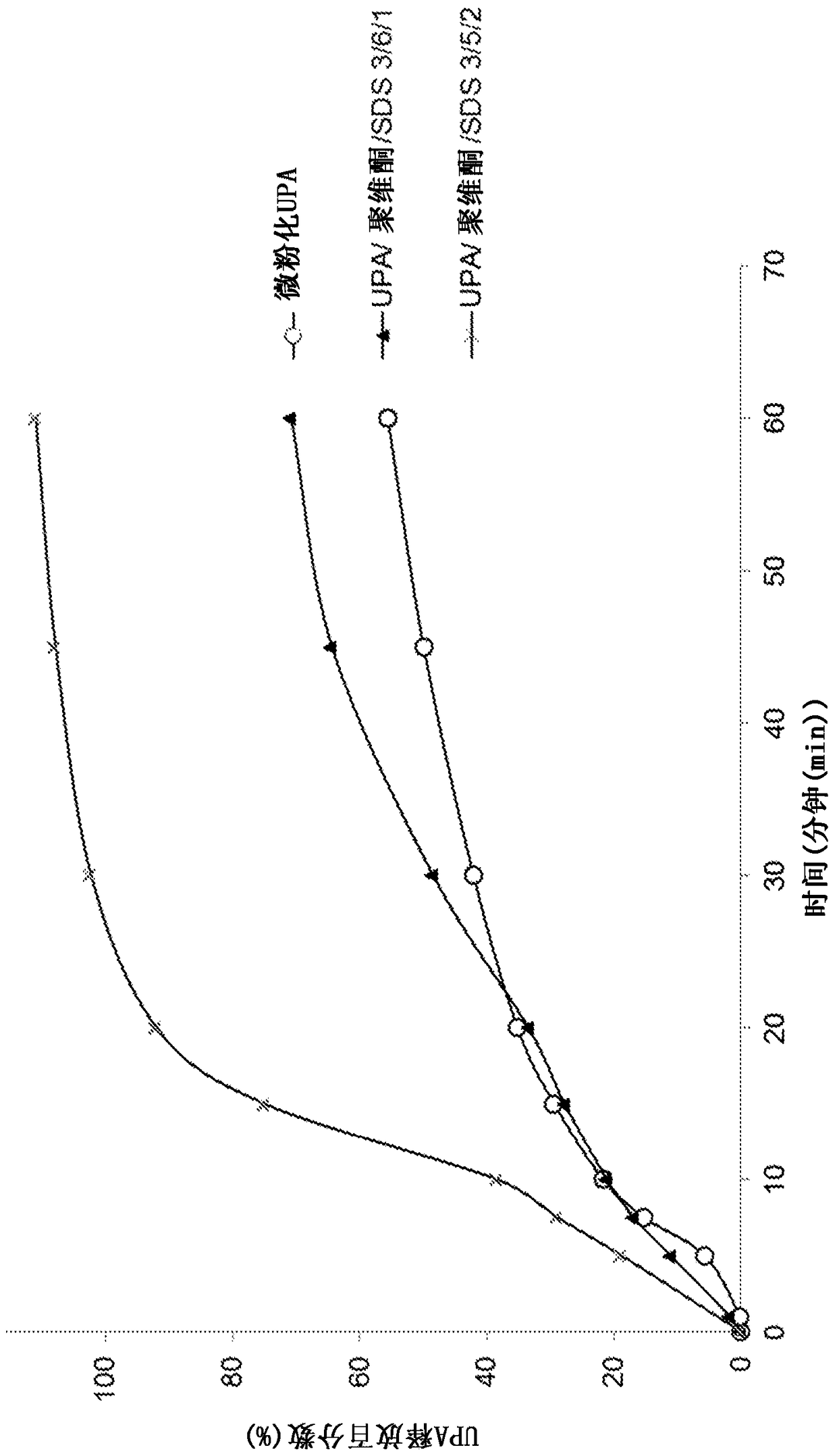
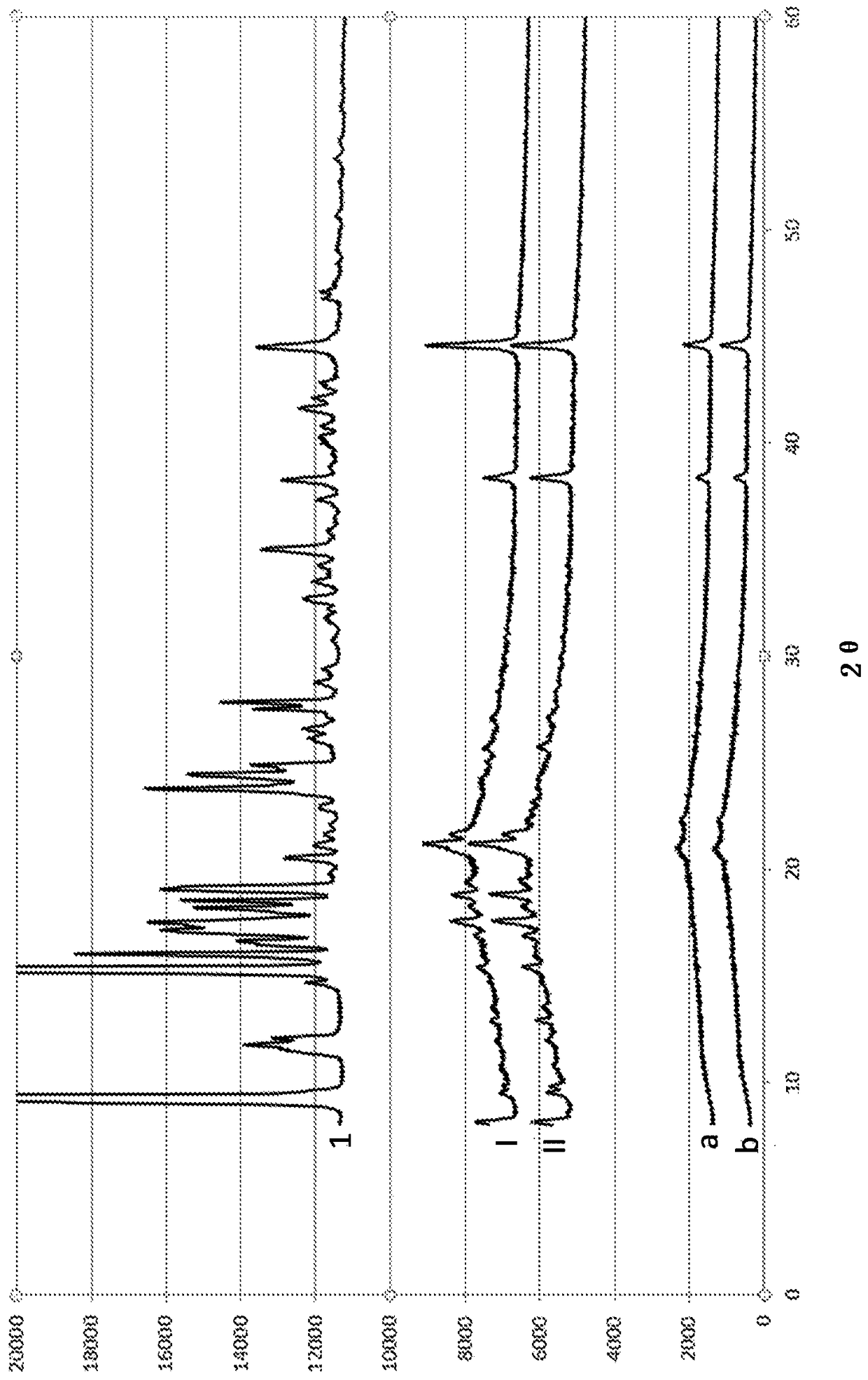
[0209] Example 1: Preparation and evaluation of various solid dispersions
[0210] 1. Preparation according to the solid dispersion of the present invention
[0211] a) Preparation of solid dispersions by means of the melting method
[0212] A solid dispersion comprising ulipristal acetate as an active ingredient and polyethylene glycol (PEG-4000) as a polymer excipient was prepared in the following manner, wherein the "polymer excipient / UPA" weight ratio was 960 / 40 :
[0213] - heat the polyethylene glycol until it is completely melted,
[0214] - Add micronized UPA to molten PEG with stirring. Heat the mixture and keep stirring until the UPA is completely dissolved,
[0215] - bring the mixture to ambient temperature with stirring,
[0216] - Optionally milling and micronizing the obtained solid dispersion to obtain the desired particle size distribution.
[0217] Other solid dispersions are also prepared by means of the melt method:
[0218] - UPA with weight ratio...
Embodiment 2
[0263] Example 2: Pharmaceutical composition integrating a solid dispersion according to the invention
[0264] Tables 3 and 4 below provide examples of pharmaceutical compositions according to the invention. These pharmaceutical compositions can be obtained by mixing the solid dispersion according to the invention with various excipients and then shaping the mixture by direct compression to obtain tablets.
[0265] Table 3: Examples of compositions according to the invention comprising 5 mg UPA
[0266]
[0267] The composition can be used, for example, in the treatment of uterine fibroids.
[0268] Table 4: Examples of compositions according to the invention comprising 30 mg UPA
[0269]
[0270] The composition is useful, for example, in emergency contraception.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com