Crystalline ledipasvir compound and process for its preparation
A technology of solvate and acetonate, which is applied in the field of medicine and achieves the effects of less degraded impurities, stable crystal form and favorable storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of Ledipavir Hemiacetone Solvate
[0029] Take 2.0 g of Radipavir diacetone hydrate, add 20 mL of n-heptane, and stir at 25-30°C for 5 hours. After filtration, the obtained solid was rinsed with n-heptane, and the solid was dried in a blast drying oven at 50° C. for 4 hours to obtain 1.6 g of light yellow solid.
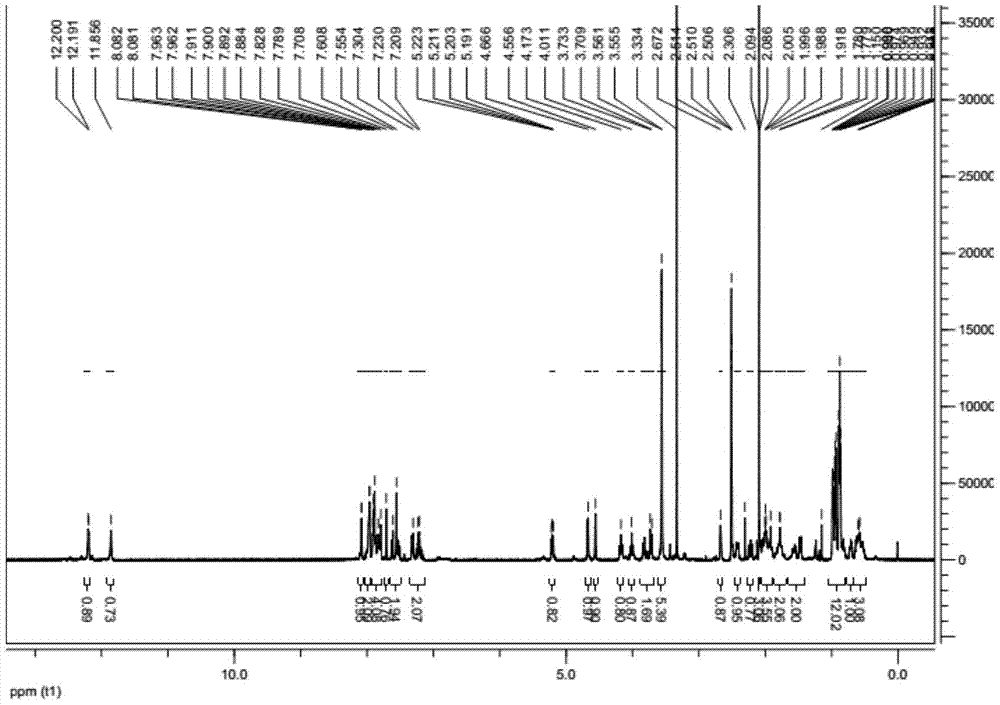
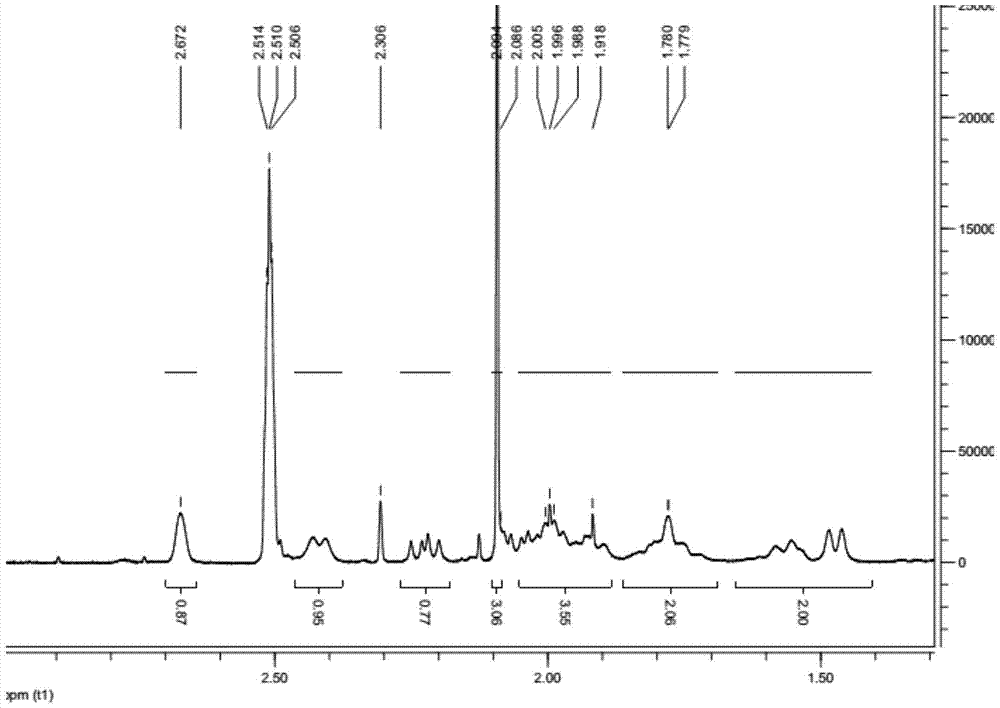
[0030] The obtained light yellow solid proton nuclear magnetic resonance spectrum detection diagram is attached figure 1 , Attached figure 2 As shown, the peak of chemical shift 2.09 is a characteristic peak of acetone, which is 3.06H after integration, indicating that acetone and Ledipavir molecules form a complex with a molecular ratio of 1:2.
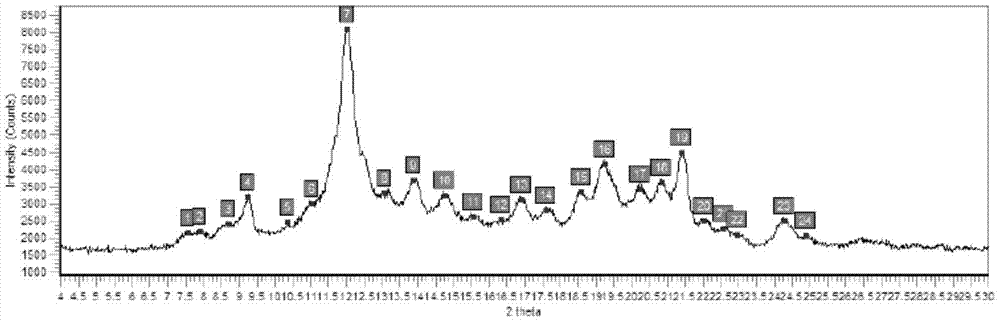
[0031] XRPD inspection chart is attached image 3 Shown.
[0032] Instruments and test conditions used for XRPD testing:
[0033] Instrument: Bruker d8 advance X-ray diffractometer
[0034] Test conditions: test current and voltage: 40kv / 40mA, detector: lyxEye, test step: 0.01°
[0035] Target type: Cu ...
Embodiment 2
[0036] Example 2: Preparation of Ledipavir Hemiacetone Solvate
[0037] Take 2.0 g of Radipavir diacetone hydrate, add 30 mL of n-hexane, and stir at 25-30°C for 4 hours. After filtration, the obtained solid was rinsed with n-hexane, and the solid was dried in a blast drying oven at 40° C. for 5 hours to obtain 1.5 g of a light yellow solid.
[0038] After testing, its proton nuclear magnetic resonance spectrum detection chart and XRPD detection chart are consistent with the product of Example 1.
Embodiment 3
[0039] Example 3: Preparation of Ledipavir Hemiacetone Solvate
[0040] Take 2.0 g of Radipavir diacetone hydrate, add 20 mL of petroleum ether, and stir at 25-30°C for 5 hours. After filtration, the obtained solid was rinsed with petroleum ether, and the solid was dried in a blast drying oven at 25° C. for 6 hours to obtain 1.6 g of light yellow solid.
[0041] After testing, its proton nuclear magnetic resonance spectrum detection chart and XRPD detection chart are consistent with the product of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com