Methods for obtaining retinal progenitors, retinal pigmented epithelial cells and neural retinal cells
A technique for retinal progenitor cells and retinal cells, applied in the field of obtaining retinal progenitor cells, retinal pigment epithelial cells and neural retinal cells, can solve the problem of low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Transplantation of human non-integrated induced pluripotent stem cells to retinal neurons and retinal pigment epithelial cells Reliable and efficient differentiation of cells
[0063] 1.1 Experimental procedure
[0064] Culture of human fibroblasts and iPS cells
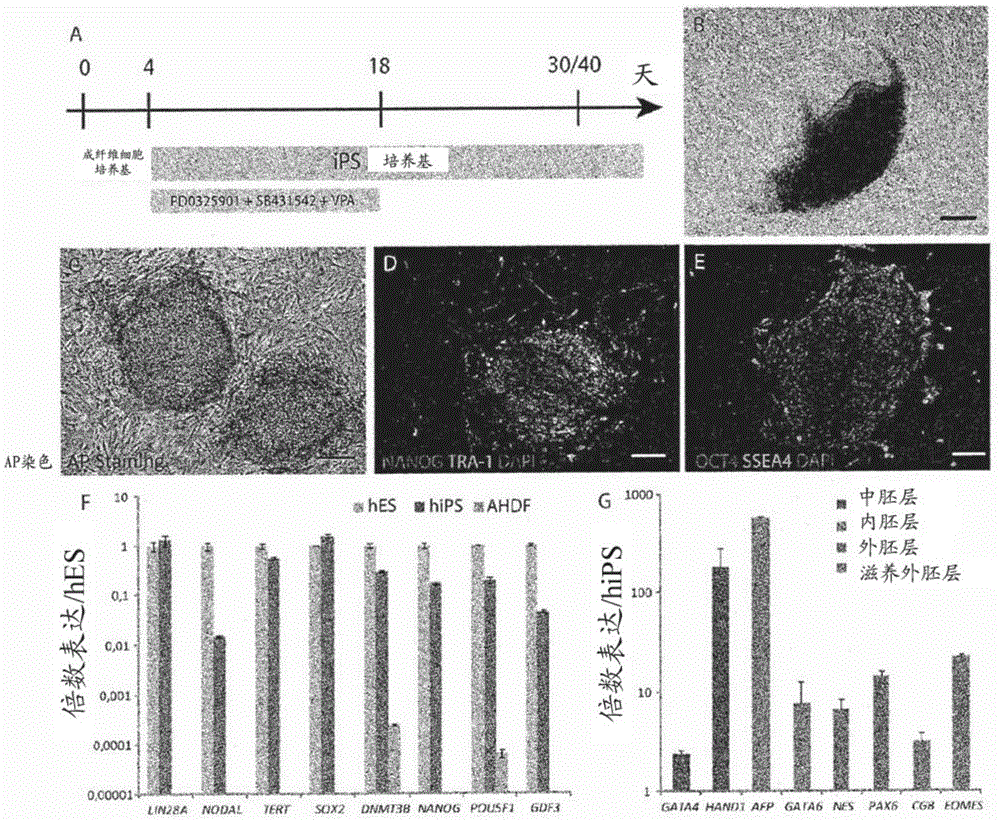
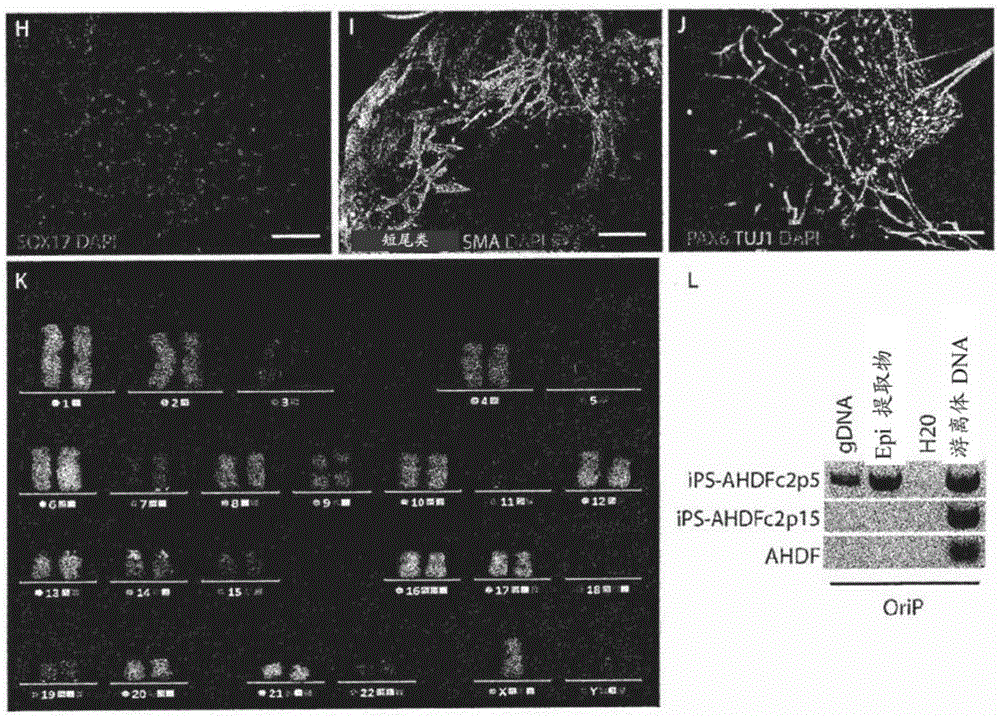
[0065] At 37°C, standard 5% CO 2 Mature human dermal fibroblasts (AHDF) from an 8-year-old boy (gifted by Dr. Rustin, INSERMU676, Paris, France) were cultured in Duchenne's modified Eagle's medium with high glucose Glutamax II ( DMEM) (Life Technologies) supplemented with 10% FBS (Life Technologies), 1 mM sodium pyruvate (Life Technologies), 1XMEM non-essential amino acids (Life Technologies). This medium is referred to as "fibroblast medium". Established human iPS cells were maintained in mitomycin-C inactivated mouse embryonic cells in ReproStem (ReproCell) medium with 10 ng / ml human recombinant fibroblast growth factor 2 (FGF2) (Preprotech) Fibroblast (MEF) trophoblast (Zenith). Cells we...
Embodiment 2
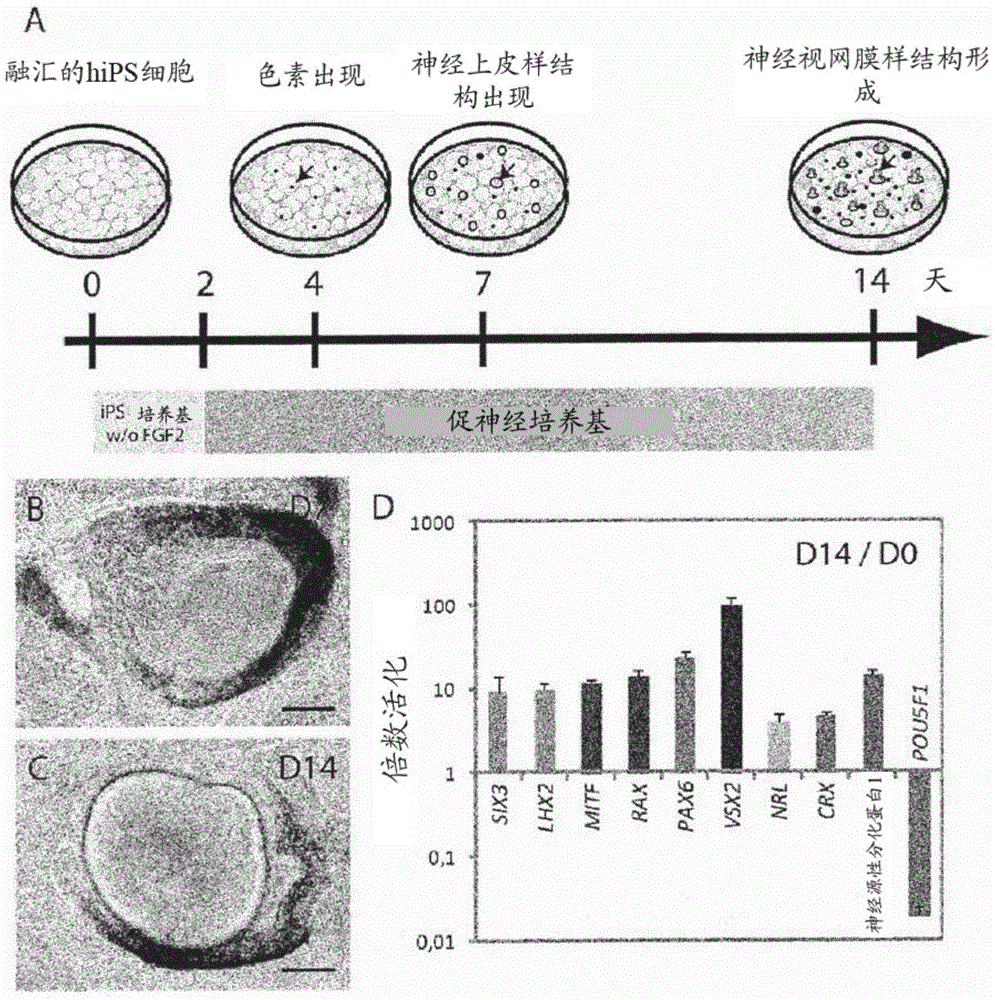
[0104] Example 2: Differentiation of retinal progenitor cells into late-born retinal cell types
[0105] Sustained maintenance of isolated NR-like structures in floating cultures, as demonstrated by qRT-PCR, allowed further differentiation of RPCs into late-born retinal cell types (Figures 9A-9E). Indeed, after first expressing premature retinal markers of mature RGC cells (BRN3A and BRN3B), amacrine cells (calretinin and GAD2), and horizontal cells (LIM) (Figure 9A and Figure 9B), it was observed that Emergence of markers of retinal cell types to late life, corresponding to cones (R / GOPSIN, BLUEOPSIN, and CONEARRESTIN) and rod photoreceptors (RHODOPSIN and RECOVERIN) (Figures 9C and 9D), bipolar cells (PKCα) ) and Müller glia (GLAST1) (Fig. 9E). Between day 21 (Fig. 9F) and day 42 (Fig. 9G), most of the NR-like structures lost their sheet-like appearance and developed OTX2-containing + , CRX + and RECOVERIN + The inner rosettes of cells, corresponding to differentiated ...
Embodiment 3
[0106] Example 3: Acceleration of Photoreceptor Precursor Generation by Notch Inhibition
[0107] Immunohistochemical analysis using antibodies against CRX and RECOVERIN demonstrated that the number of photoreceptor precursors varied from day 14 (Figure 3J) to day 28 (Figure 3L, Figure 5 B) gradually increased. Between days 21 and 35, NR-like structures lost their lamellar structure and developed internal rosettes containing CRX and RECOVRIN positive cells ( Figure 5 B). Interestingly, on day 21, continuous addition of the Notch inhibitor DAPT for 7 days was sufficient to significantly increase the number of CRX-positive cells and RECOVRIN-positive cells ( Figure 5 B). Subsequent treatment with DAPT also resulted in a large increase in the number of cells expressing CRX and RECOVERIN from day 28 to day 35 ( Figure 5 B). One week of treatment with DAPT increased photoreceptor precursor production between days 21 and 28, as CRX at day 28 increased the production of photor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com