Shuttle plasmid and construction method and application thereof
A technology for shuttle plasmids and plasmids, applied in the biological field, can solve the problems of low conjugation transfer efficiency, large plasmid size, inability to stably exist Riemerella anatipestifer, and achieve the effect of high conjugation transfer efficiency and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] According to the genetic background of Riemerella anatipestifer, a replication initiation gene capable of replicating in Riemerella anatipestifer is designed, and the specific sequence is shown in SEQ ID NO.1.
[0027] Then design the primers for amplifying SEQIDNO.1 according to the synthesized replication initiation gene sequence. For the convenience of vector construction, add HindⅢ and pstI restriction sites respectively at the upstream and downstream of the primers. The specific primers are as follows:
[0028] Upstream primer: RARepP15'-ccc aagctt gggctatttaggcattagccctc-5' (SEQ ID NO. 2);
[0029] There are primers below: RARepP25'-aaaa ctgcag ccaatgcattggaacagatctcgtatagagctcg-5' (SEQ ID NO. 3);
[0030] Using the synthesized SEQIDNO.1 as a template, SEQIDNO.2 and SEQIDNO.3 as primers for PCR amplification, the PCR amplification program was pre-denaturation at 98°C for 30s; denaturation at 98°C for 10s, annealing at 55°C for 30s, extension at 72°C for 1min30...
Embodiment 2
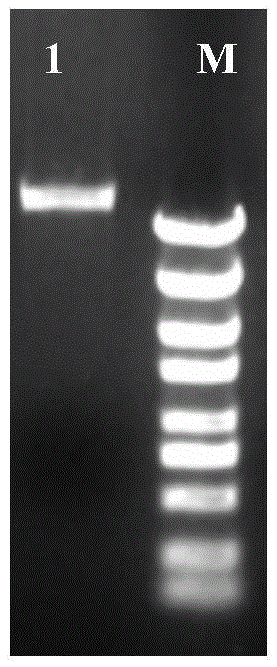
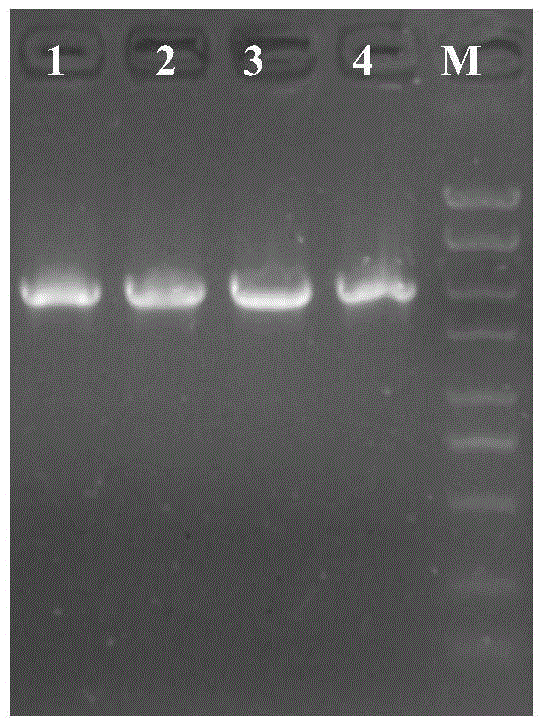
[0033] The shuttle plasmid pMY02 constructed in Example 1 was transformed into the donor strain Escherichia coli S17-1, and after 10 generations, the plasmid was extracted to detect the stability of the shuttle plasmid pMY02. The electrophoresis results were as follows: image 3 shown. The results showed that the structure of the shuttle plasmid pMY02 was stable after 10 passages, indicating that the shuttle plasmid pMY02 had good stability in Escherichia coli.
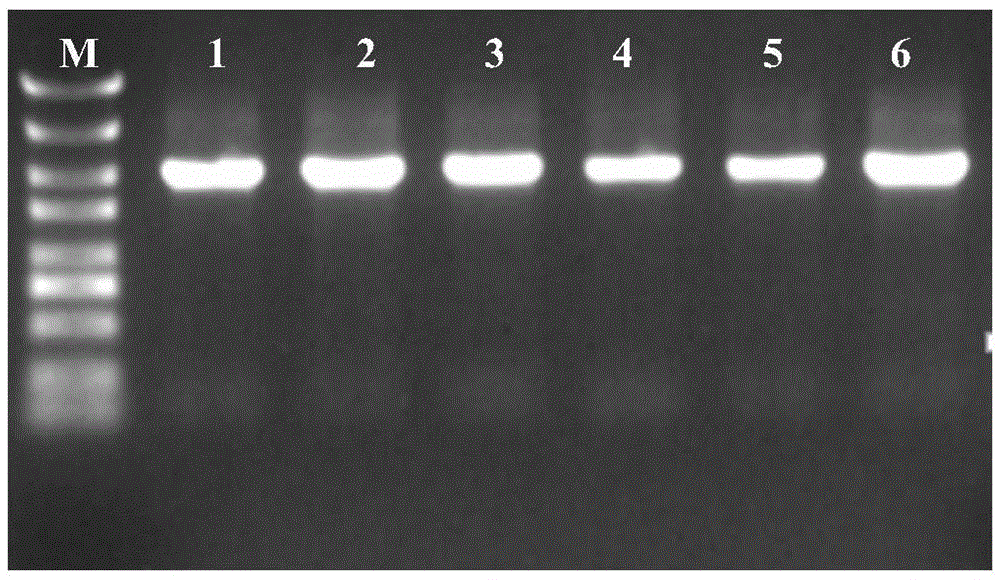
[0034] Then transfer the shuttle plasmid pMY02 to Riemerella anatipestifer RAATCC11845 by conjugative transfer method to obtain RAATCC11845::pMY02, then use 1 μg / ml of cefoxitin to screen positive clones, and then the screened bacterial strain RAATCC11845::pMY02 in the blood Upload 10 generations on the plate, and finally use SEQIDNO.2 and SEQIDNO.3 as primers for PCR detection, the results are as follows Figure 4 shown. The results showed that the structure of the pMY02 recombinant plasmid was stable after 10 pass...
Embodiment 3
[0036] After constructing the shuttle plasmid pMY02, the Riemerella anatipestifer TonB1 gene sequence was inserted into the NcoI and XbaI restriction sites of the shuttle plasmid pMY02, and the primers for TonB1 gene amplification were as follows:
[0037] Upstream primer: TonB1CompP1: 5'-catg ccatggatgagccaaaccataaatac-3' (SEQ ID NO. 4);
[0038] Downstream primer: TonB1CompP2: 5'-ctag tctaga ttagaaagtgattttgtaagtgcctg-3' (SEQ ID NO. 5);
[0039] Using the Riemerella anatipestifer genome as a template, and the sequences shown in SEQIDNO.4 and SEQIDNO.5 as primers, the PCR amplification conditions were pre-denaturation at 98°C for 30s; denaturation at 98°C for 10s, annealing at 50°C for 30s, and extension at 72°C for 30s. Cycle 30 times; then extend at 72°C for 10 minutes; finally store at 16°C. The amplified products were subjected to agarose gel electrophoresis, and the results were as follows: Figure 5 shown. The results showed that TonB1 was amplified, and the band...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com