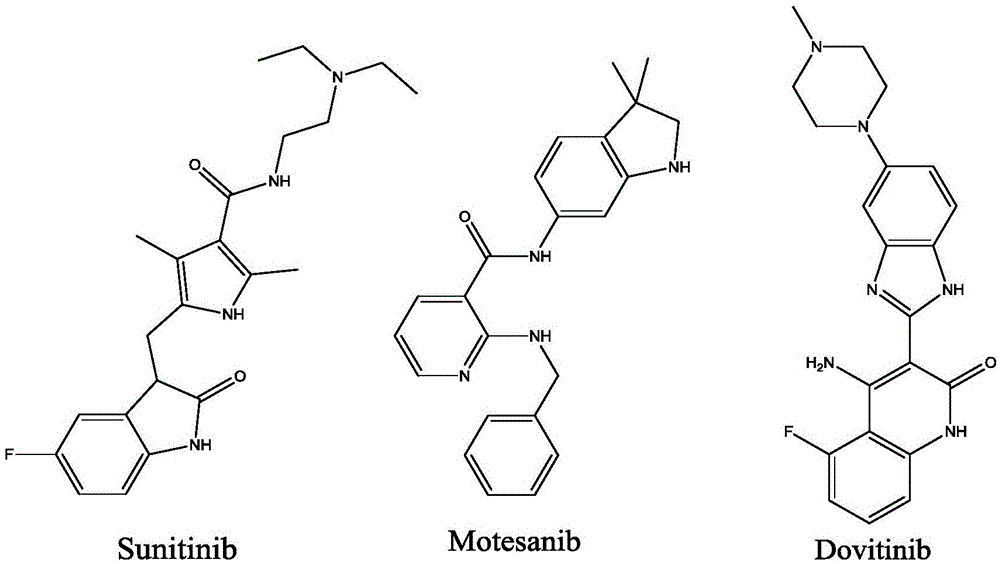
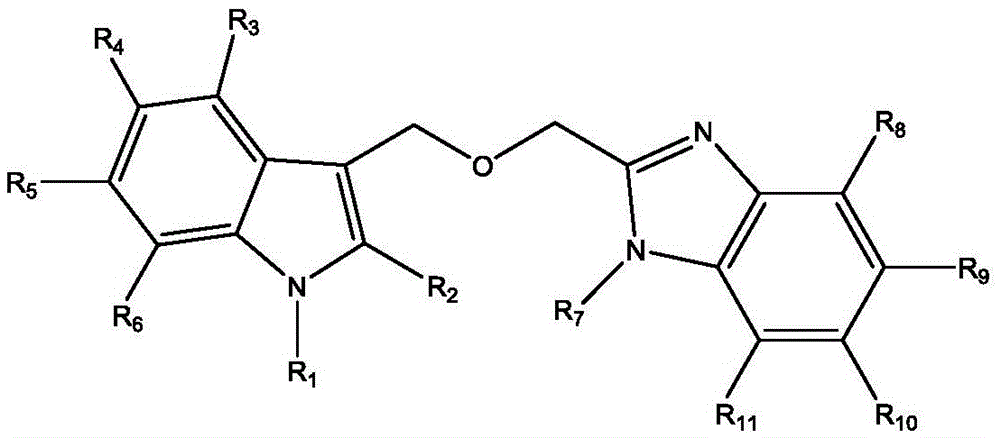
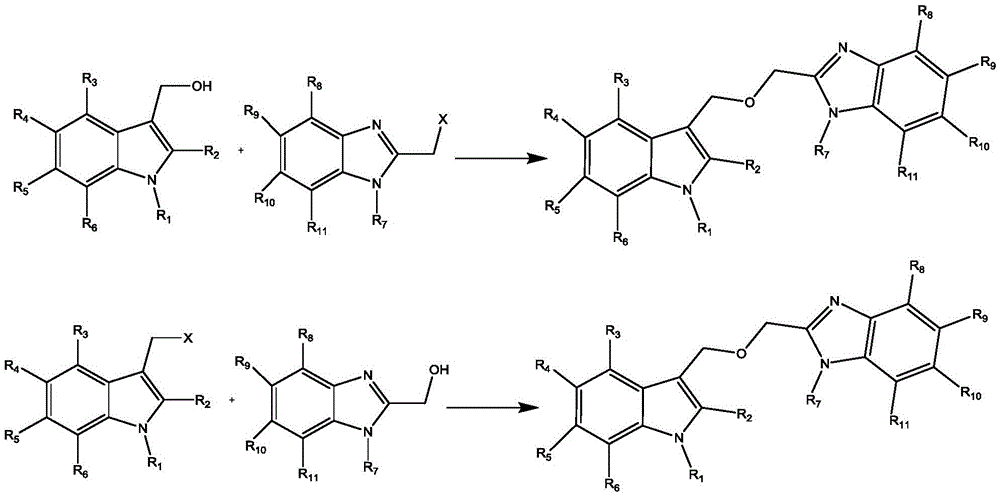
2-(((1H-indole-3-yl) methoxyl) methyl)-1H-benzimidazole derivatives and preparation thereof
A technology of benzimidazoles and derivatives, which is applied in the field of medicinal chemistry and can solve the problems of few types of compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Add 1.19g of 1-benzylindole-3-methanol and 10ml of tetrahydrofuran to a 50ml three-necked flask, start stirring, slowly add 10ml of tetrahydrofuran solution containing 0.83g of 2-chloromethylbenzimidazole dropwise at room temperature, and heat up to At 60°C, add 1.0 g of potassium carbonate powder and react for 4 hours. After the reaction is complete, filter, concentrate the filtrate, and separate by column chromatography to obtain 1.15g of 2-(((1-benzyl-1H-indol-3-yl)methoxy)methyl)-1H-benzimidazole , yield 62.7%.
Embodiment 2
[0016] Example 2: Add 1.19g of 1-benzylindole-3-methanol and 10ml of dioxane to a 50ml three-necked flask, start stirring, and slowly add 1.06g of 2-chloromethyl-5-nitrobenzimidazole dropwise at room temperature 10ml of dioxane solution, heated up to 80°C, added 1.0g of potassium carbonate powder, and reacted for 4h. After the reaction is complete, filter, concentrate the filtrate, and separate by column chromatography to obtain 1.11g of 2-(((1-benzyl-1H-indol-3-yl)oxy)methyl)-5-nitro-1H - Benzimidazole, yield 53.9%.
Embodiment 3
[0017] Example 3: Add 0.94g of 1-allylindole-3-methanol and 10ml of N,N-dimethylformamide to a 50ml three-necked flask, start stirring, and slowly add 0.82g of 2-chloromethyl-5 - 10ml of N,N-dimethylformamide solution of methylbenzimidazole, heated up to 100°C, added 1.0g of potassium carbonate powder, and reacted for 5h. After the reaction is complete, filter, concentrate the filtrate, and separate by column chromatography to obtain 1.15g of 2-(((1-allyl-1H-indol-3-yl)methoxy)methyl)-5-methyl -1H-benzimidazole, yield 69.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com