Intrauterine delivery system
A delivery system, uterine technology, applied in the field of improved intrauterine delivery system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Core preparation:
[0054] In an internal mixer, 65 parts by weight of 18-methyl-15β, 16β-methylene-19-nor-20-spiro-4-en-3-one and 35 parts by weight of poly(dimethyl Silicone) elastomer blend. The poly(dimethylsiloxane) elastomer used for the drug reservoir portion is a silicone-based filler-free PDMS (dimethylvinyl-terminated poly[dimethyl-co-methylvinyl]silicone Oxane) material crosslinked by a hydrosilylation reaction using platinum as a catalyst and poly(dimethyl-co-methylhydrogensiloxane) as a crosslinking agent. The drug-containing mixture was extruded into a tubular form with a wall thickness of 0.8 mm and an outer diameter of 2.8 mm, and heat cured while crosslinking occurred. The cross-linked cores were cut into lengths of 5 and 8 mm.
[0055] Preparation of the membrane of the "slower release" part (reservoir 2):
[0056] The elastomers used in the film were two silicone elastomers containing silica fillers - PDMS (Dimethylvinyl-terminated poly[dimethyl-c...
Embodiment 2
[0061] Embodiment 2: drug release test
[0062] method:
[0063] The release rate of drug from the IUS was measured in vitro as follows:
[0064] The intrauterine delivery system was secured in a longitudinal orientation in a stainless steel holder, and the holder and device were placed together in a glass vial containing 75 ml of dissolution medium. The glass vials were shaken in a shaking water bath at 37°C, 70 strokes / minute. The dissolution medium was withdrawn and replaced with fresh dissolution medium at predetermined time intervals and analyzed for the amount of released drug using standard HPLC methods. The concentration of the dissolution medium and the timing of changing (withdrawing and replacing) the medium are chosen to maintain sink conditions during the test.
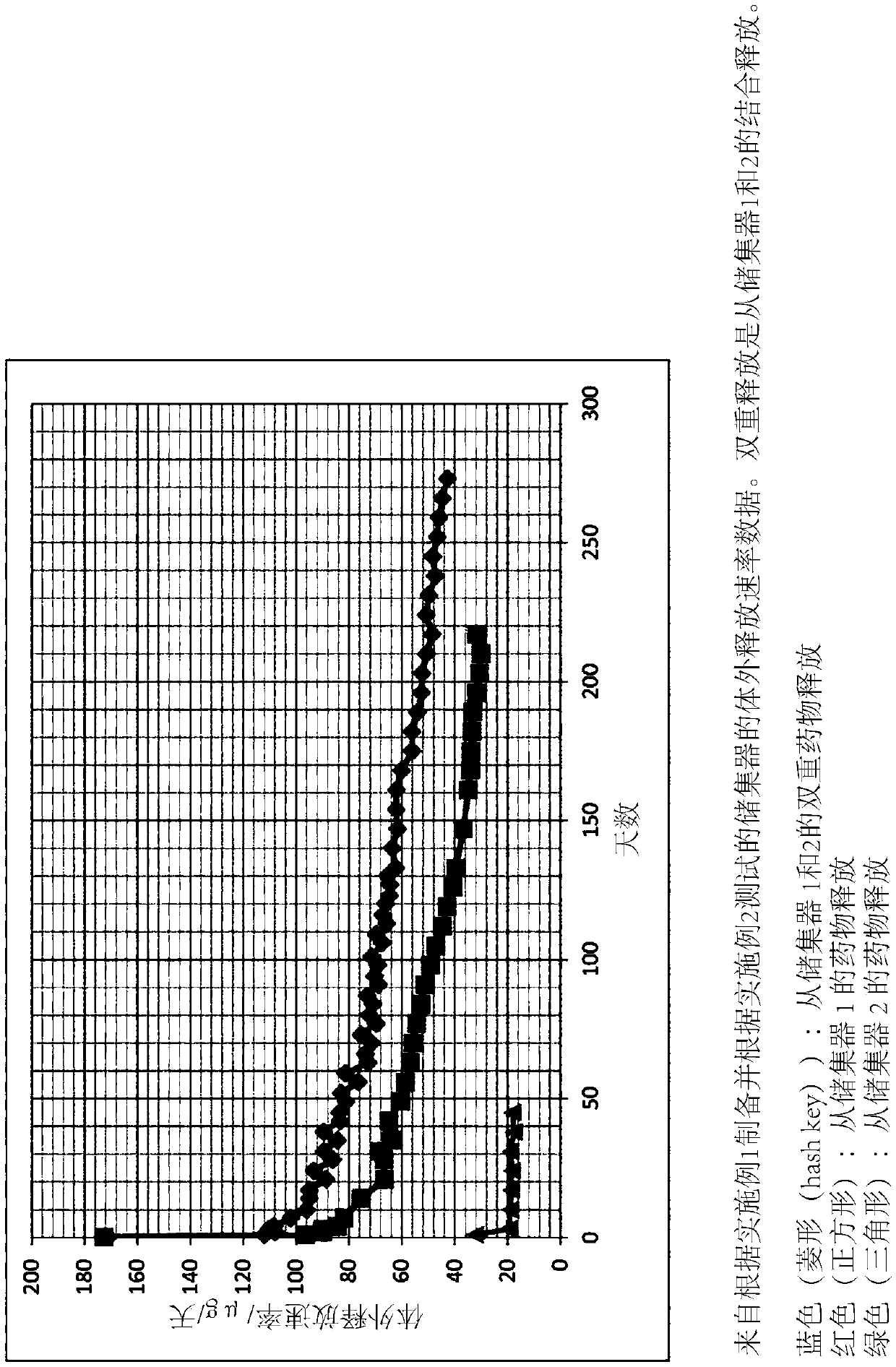
[0065] Results: The release rates obtained from the individual fractions and the combined system were in Figure 1 / 7 shown in . It can be seen that the rate of release from the reservoir containing...
Embodiment 3
[0069] Comparative study in monkeys: Vehicle vs 2, 5 μg / day LNG vs 2, 5 μg / day NP Method:
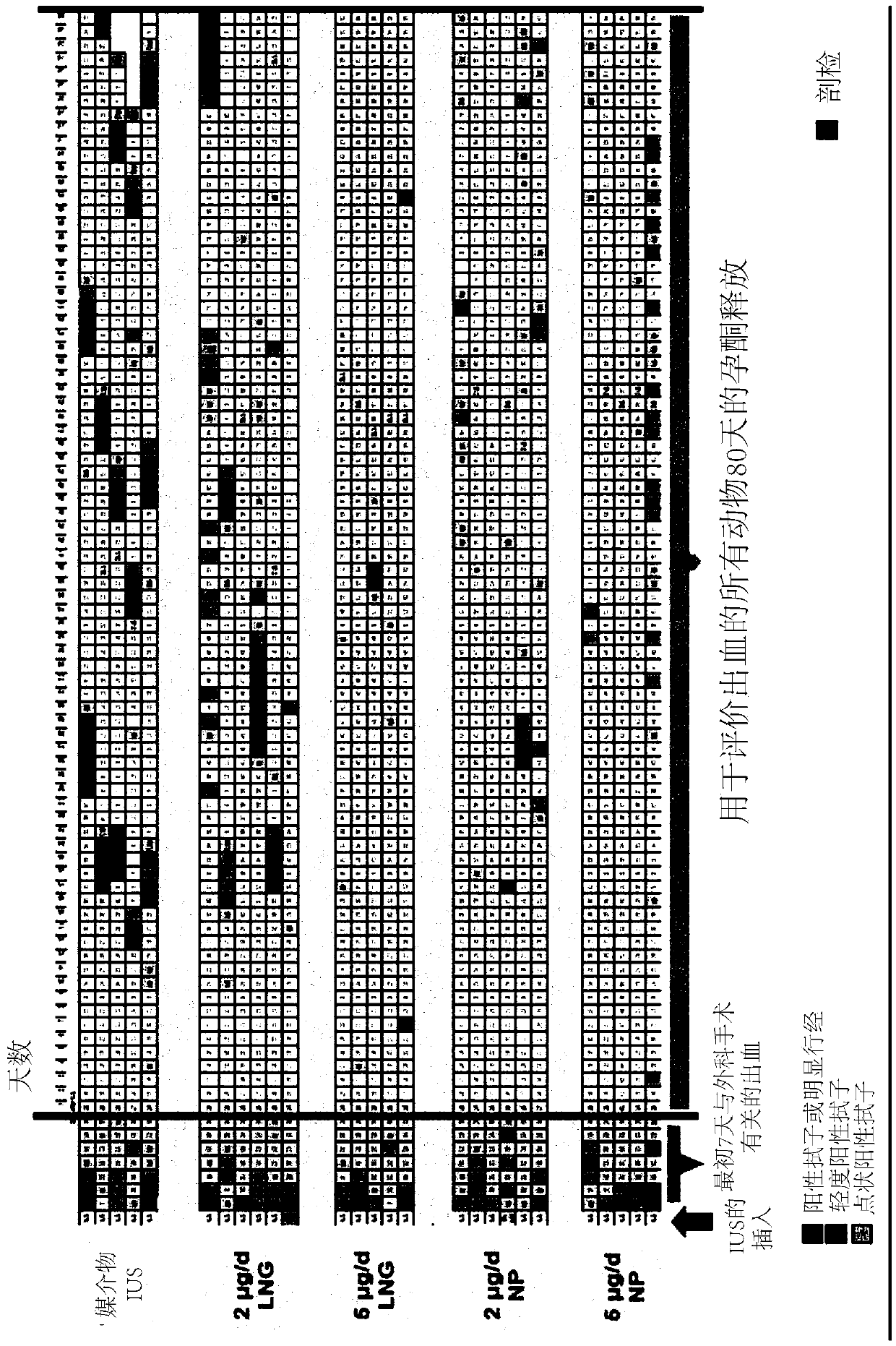
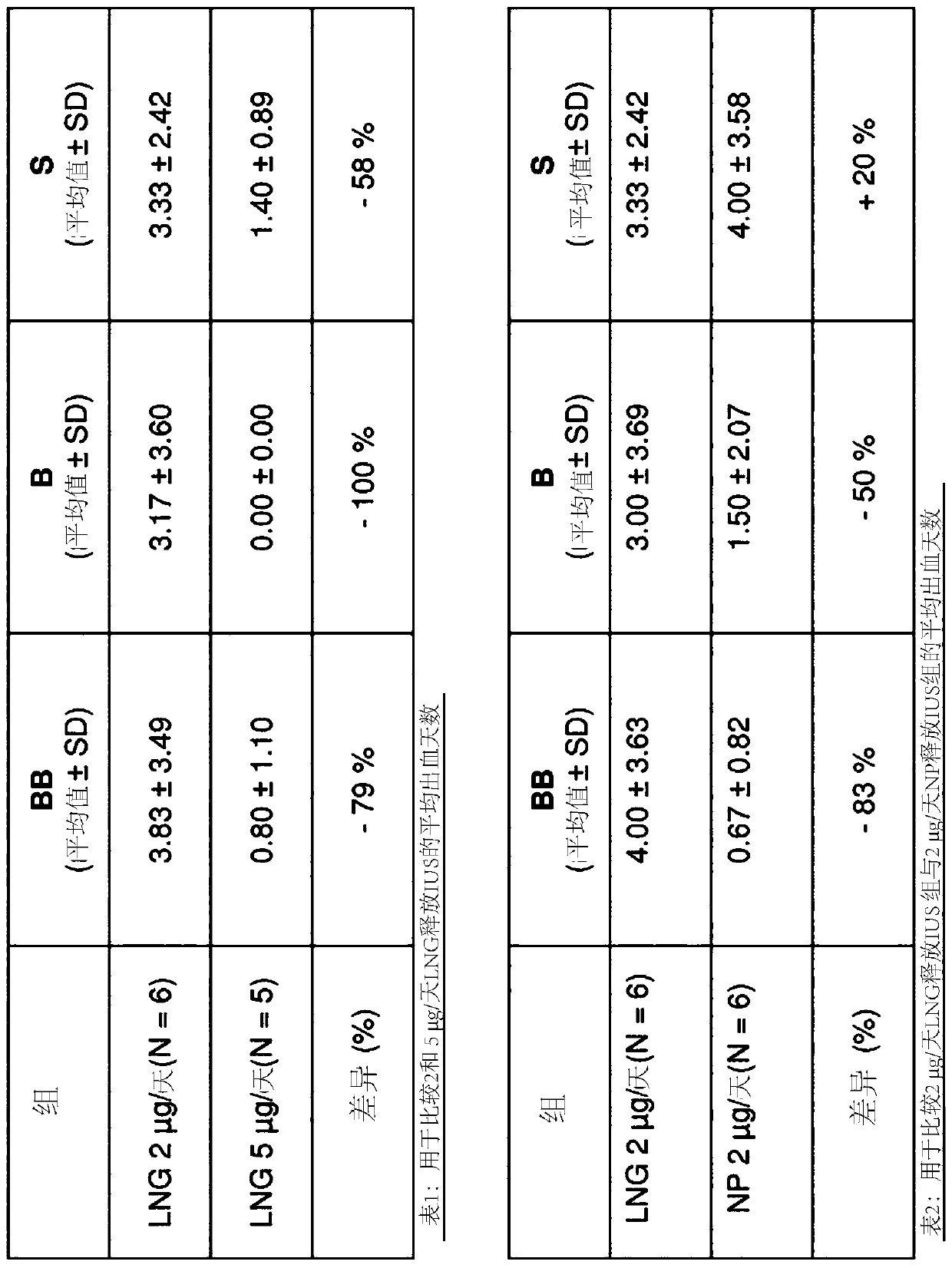
[0070] Animal Handling: Adult non-pregnant cynomolgus monkeys were monitored for regular menstrual cycles. Uterine bleeding was assessed daily by vaginal swab (for occasional vaginal spotting) and menstrual blood loss by vaginal plug. After 2 menstrual cycles (~60 days), animals were divided into treatment groups, laparotomy was performed on days 6-8 (ideally day 7) of the follicular phase, an IUS was inserted into the uterine cavity via hysterotomy and original A bit of stitching. IUS treatment was as follows (n=5-6 / group):
[0071] Group 1: Vehicle IUS
[0072] Group 2: 2 μg / day LNG
[0073] Group 3: 5 μg / day LNG
[0074] Group 4: 2 μg / day NP
[0075] Group 5: 5 μg / day NP
[0076] Grading of Bleeding: Bleeding is classified into three grades: a) positive swab or frankenses, which is the most severe form of bleeding (BB, red), b) mildly positive swab, which is a moderate type of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com