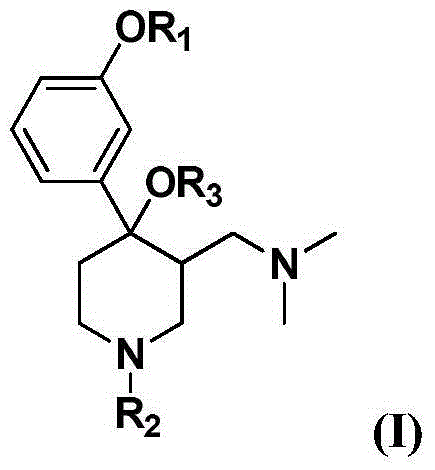
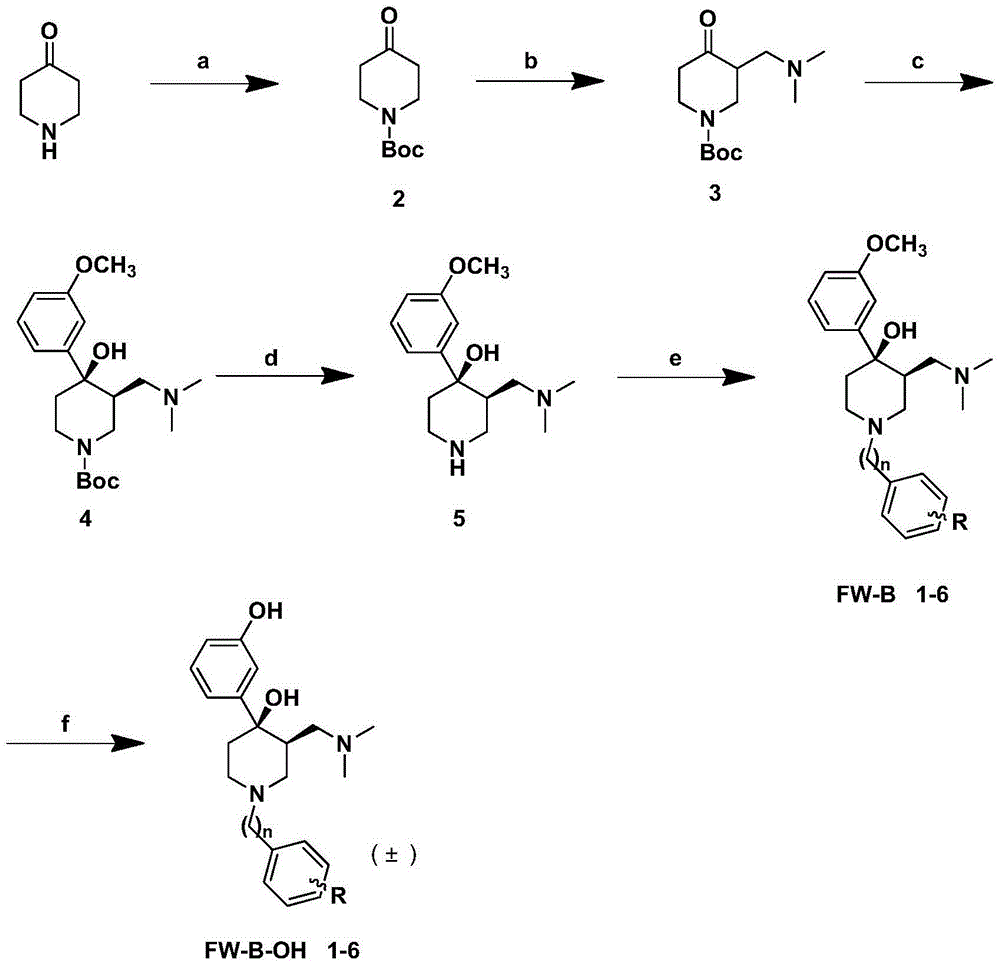
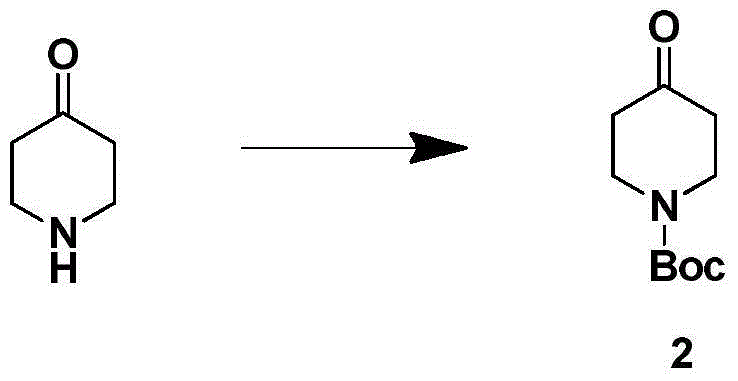
Aminomethylpiperidine derivatives, and preparation method and pharmaceutical application thereof
A technology of aminomethylpiperidine and derivatives, which is applied in the field of medicine for diseases, and can solve the problems of clinical application limitations, respiratory depression and addictive side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084]
[0085] 1-Benzyl-3-((dimethylamino)methyl)-4-(3-methoxyphenyl)-piperidin-4-ol
[0086] Preparation of compound FW-B-1
[0087] According to general procedure 5, using compound 5 as raw material, and benzyl bromide, K 2 CO 3 A nitrogen atom alkylation reaction occurs to obtain compound FW-B-1. 1 HNMR (400MHz, DMSO-d 6)δ11.78(s,1H),7.73(s,2H),7.49(s,3H),7.34(t,J=7.8Hz,1H),7.06(d,J=6.9Hz,2H),6.88( d,J=8.1Hz,1H),5.85(s,1H),4.39(t,J=14.2Hz,2H),3.77(s,3H),3.17(d,J=8.5Hz,3H),3.05– 2.92(m,1H),2.68–2.35(m,10H),1.79(d,J=15.1Hz,1H). 13 CNMR (101MHz, DMSO-d 6 )δ159.28, 146.76, 131.60, 129.57, 129.46, 129.27, 128.74, 116.93, 112.35, 111.18, 70.85, 58.66, 55.00, 54.89, 51.12, 46.09, 44.14, 37.84, 36.26.
[0088] ESI-MSm / z355.3[M+H] + HRMSm / zcalcdforC 22 h 30 NO 2 [M+H]+, 340.2271; found, 340.2281.
Embodiment 2
[0090]
[0091] 1-Phenylethyl-3-((dimethylamino)methyl)-4-(3-methoxyphenyl)-piperidin-4-ol
[0092] Preparation of compound FW-B-2
[0093] According to general operation method 5, with compound 5 as raw material, with bromophenylethane, K 2 CO 3 A nitrogen atom alkylation reaction occurs to obtain compound FW-B-2. 1 HNMR (400MHz, DMSO-d 6 )δ11.40(s,1H),10.61(s,1H),7.42–7.34(m,3H),7.29(dd,J=13.9,7.0Hz,3H),7.11(s,1H),7.07(d ,J=7.8Hz,1H),6.90(dd,J=8.1,2.3Hz,1H),5.94(s,1H),4.29(d,J=10.9Hz,1H),3.79(s,3H),3.59 –3.40(m,2H),3.30(dd,J=11.9,6.5Hz,4H),3.18(dt,J=17.3,9.4Hz,2H),3.05–2.91(m,1H),2.73–2.56(m ,4H),2.50–2.42(m,4H),1.85(d,J=14.7Hz,1H). 13 CNMR (101MHz, DMSO-d 6 )δ159.81, 147.32, 137.60, 130.13, 129.18, 129.14, 127.29, 117.41, 112.85, 111.69, 71.41, 57.16, 55.74, 55.52, 51.02, 49.00, 44.78, 38.57 / IMS.29.02 +H] + .
Embodiment 3
[0095]
[0096] 1-phenylpropyl-3-((dimethylamino)methyl)-4-(3-methoxyphenyl)-piperidin-4-ol
[0097] Preparation of compound FW-B-3
[0098] According to general operation method 5, with compound 5 as raw material, with bromophenylpropane, K 2 CO 3 A nitrogen atom alkylation reaction occurs to obtain compound FW-B-3. 1 HNMR (400MHz, DMSO-d 6 ) 1 HNMR(400MHz,DMSO)δ11.18(s,1H),10.24(s,1H),7.42–7.16(m,6H),7.15–6.99(m,2H),6.89(dd,J=8.1,2.2Hz ,1H),5.89(s,1H),4.02(d,J=11.2Hz,1H),3.77(s,3H),3.46(dd,J=16.3,9.2Hz,1H),3.24(dd,J= 22.4,10.1Hz,3H),3.09(t,J=10.7Hz,2H),3.01–2.85(m,1H),2.79–2.56(m,6H),2.43(t,J=8.6Hz,4H), 2.17(d,J=7.1Hz,2H),1.80(d,J=14.6Hz,1H). 13 CNMR (101MHz, DMSO-d 6 )δ159.27,146.80,140.55,129.60,128.40,128.32,126.09,116.91,112.32,111.18,70.89,55.71,55.29,54.99,50.61,48.39,44.26,37.98,36.42,32.12,25.08.ESI-MSm / z383.3 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com