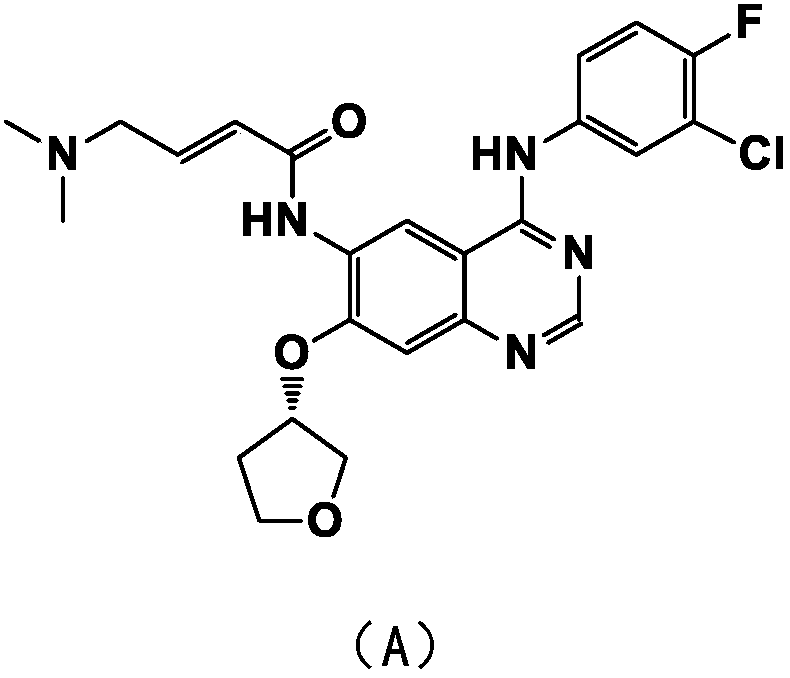
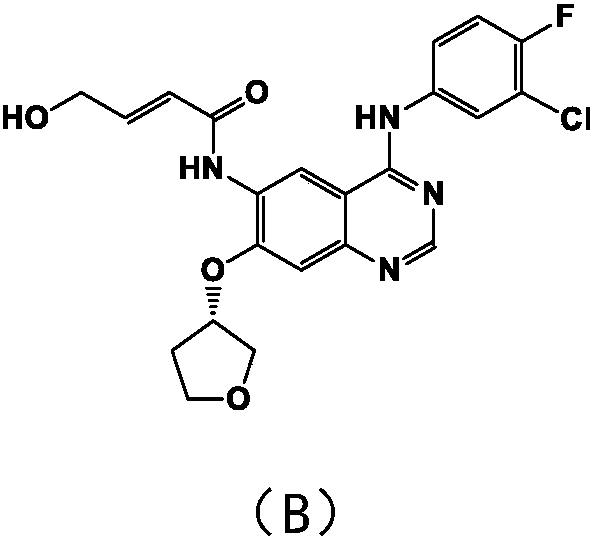
A kind of refining method of afatinib
A refining method and afatinib technology, which are applied in the refining field of afatinib, can solve the problems that afatinib dimaleate cannot meet the requirements for medicinal use, etc., and achieve good impurity removal effect and ease of use. Operation, effect of low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] This reference example is used to illustrate the preparation method of afatinib crude product. Specific steps are as follows:
[0025] a. Add 6.49kg of carbonyldiimidazole to 15.0L of tetrahydrofuran, stir, raise the temperature to 40±2°C, add 7.85kg of diethylphosphonoacetic acid and 7.5L of tetrahydrofuran solution dropwise, and keep stirring at 35-40°C for 0.5- 1h, obtain solution A, standby;
[0026] b. 7.5kg of N-4-(3-chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine Add to 30.0L tetrahydrofuran, heat up to 0±2°C, add the pre-prepared solution A; keep stirring at 30±2°C for 2 hours, after the reaction is complete, add 75.0L methyl tert-butyl ether, stir and crystallize at room temperature overnight, centrifuge, Use 20L of tetrahydrofuran / methyl tert-butyl ether mixed solution (1:1) and 20.0L of purified water to wash the solid successively, and dry to obtain (S)-diethyl(2-((4-((3-chloro-4 -Fluorophenyl)amino)-7-((tetrahydro-3-furyl)o...
Embodiment 1
[0030] Take 300g of the crude product of afatinib prepared in the reference example, add it to a mixed solution of 300mL of anhydrous methanol and 300mL of acetone, heat up to 50°C, stir to dissolve, slowly add 3.0L of methyl tert-butyl ether, at 50°C Insulated and stirred for 30 minutes, cooled to 25°C for 2 hours, crystallized at 25°C for 3 hours, centrifuged, washed the solid with 500 mL of methyl tert-butyl ether, and dried to obtain 239 g of afatinib as a white solid.
[0031] The purity of the obtained solid was 99.737%, the impurity H content was 0.048%, the largest unknown monomer impurity content was 0.076%, and the total impurity content was 0.263%.
Embodiment 2
[0033] Take 1.00 kg of the crude product of afatinib prepared in the reference example, add it to a mixed solution of 1.0 L of anhydrous methanol and 1.0 L of acetone, raise the temperature to 50 ° C, stir and dissolve, and slowly add 10.0 L of methyl tert-butyl ether, Stir at 50°C for 30min, cool down to 20-30°C for 1h, crystallize at 20-30°C for 2h, centrifuge, rinse the solid with 1.0L of methyl tert-butyl ether, and dry to obtain 826g of afatinib as a white solid.
[0034] The obtained solid had a purity of 99.878%, an impurity H content of 0.023%, a maximum unknown monomer impurity content of 0.050%, and a total impurity content of 0.122%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com