9-amino-oxidized isoapophine-platinum (ii) complex and its synthesis method and application
A synthesis method and compound technology, applied in the field of medicine, can solve the problems such as the synthesis method and application of platinum (II) complexes that have not yet been discovered, the destruction of the aromatic planarity of the oxidized isoaporphine parent ring, and the influence of electron cloud density on active sites. , to achieve significant in vitro anti-tumor activity, good medicinal value, and obvious inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
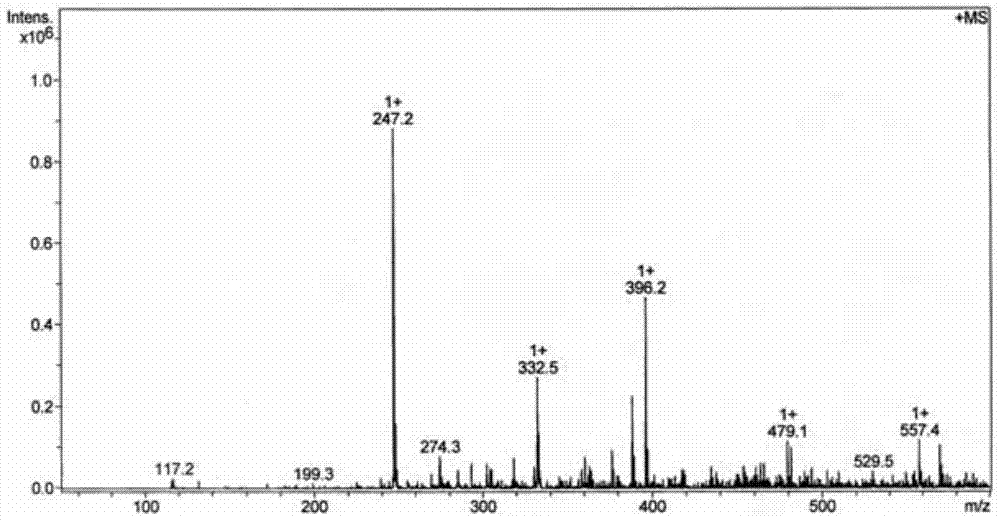
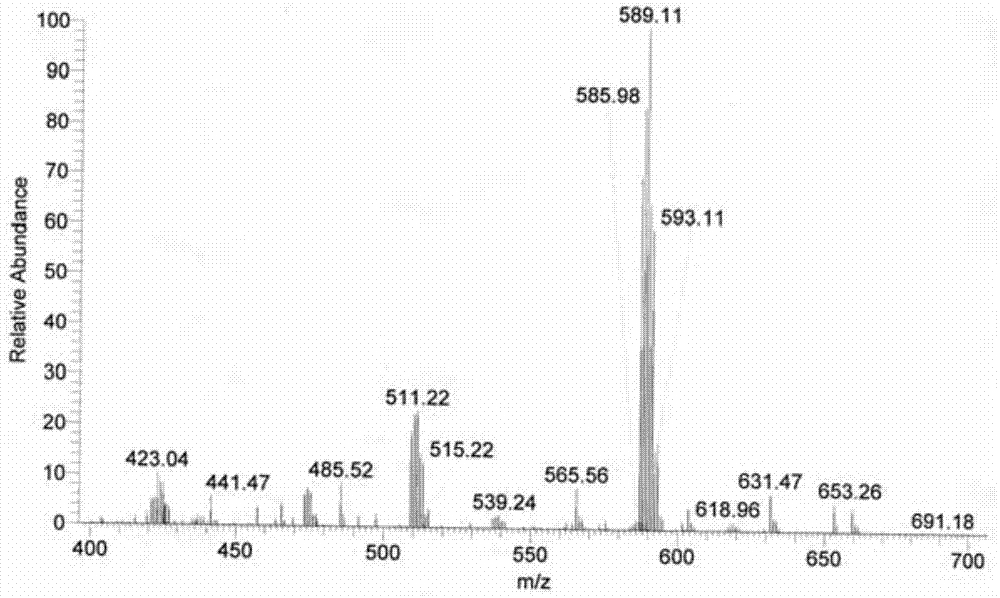
[0041] Accurately weigh 0.5 mmol of ligand L and 0.5 mmol of dichlorobis(dimethylsulfoxide) platinum (II), dissolve ligand L in 55 mL of 70v / v% methanol and acetonitrile (volume of methanol and acetonitrile ratio of 1:1)), dichlorobis(dimethyl sulfoxide) platinum (II) was dissolved in 2 mL of dimethyl sulfoxide and acetone (the volume ratio of dimethyl sulfoxide and acetone was 3 : 1) in the mixed solution, two kinds of solutions were mixed, and reacted 24 hours at 65 ℃, after concentrating and evaporating to remove most of solvent (85% of solvent add-on), be cooled to room temperature and stand still, separate out yellow solid product (productivity 95%).
[0042] The resulting yellow solid product is identified:
[0043] (1) Infrared spectrum, the specific spectrum characterization data are as follows:
[0044] IR(KBr):3527,3216,3110,2917,1653,1601,1494,1439,1396,1299,1222,1131,1026,977,920,843,768,716,655,590,503,441cm -1 .
[0045] (2) Proton NMR spectrogram, the specif...
Embodiment 2
[0055] The amount of accurately weighed substance is 0.5mmol of ligand L and 0.4mmol of dichlorobis(dimethylsulfoxide) platinum (II), and the ligand L is dissolved in 35mL of 85v / v% methanol and acetonitrile (methanol In the mixed solution with acetonitrile volume ratio of 20:80), dichlorobis(dimethylsulfoxide) platinum(II) was dissolved in 5mL of acetone, and the two solutions were mixed and reacted at 80°C for 48 hours , concentrated and evaporated to remove most of the solvent (90% of the added amount of solvent), cooled to room temperature and stood still, and the yellow solid target product (yield 80%) was precipitated.
Embodiment 3
[0057] The amount of accurately weighed substance is 0.5mmol of ligand L and 0.55mmol of dichlorobis(dimethylsulfoxide) platinum (II), and the ligand L is dissolved in 43mL of 60v / v% methanol and acetonitrile (methanol In the mixed solution with acetonitrile volume ratio of 0.6:99.4), dichlorobis(dimethylsulfoxide)platinum(II) was dissolved in 1mL of dimethylsulfoxide, the two solutions were mixed, and heated at 60°C The reaction was carried out for 12 hours, concentrated and evaporated to remove most of the solvent (75% of the added amount of solvent), cooled to room temperature and allowed to stand, and the target product was precipitated as a yellow solid (yield: 85%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com