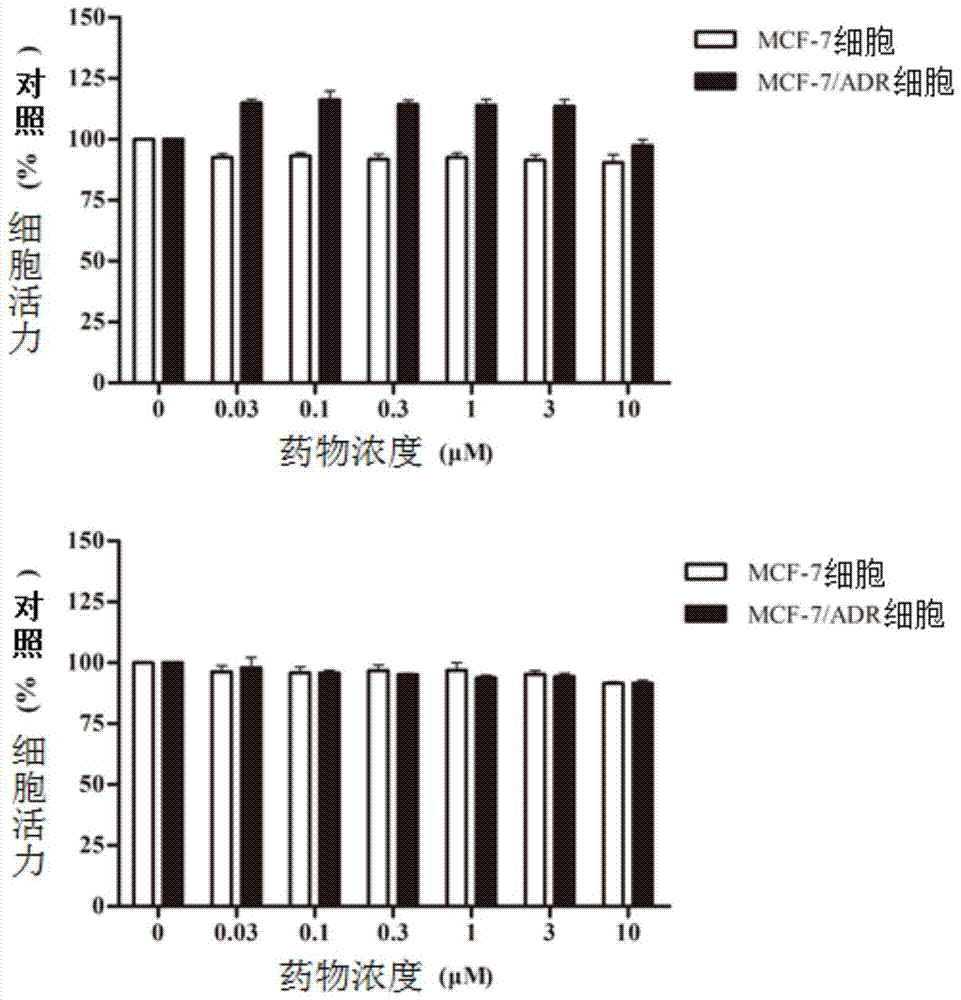
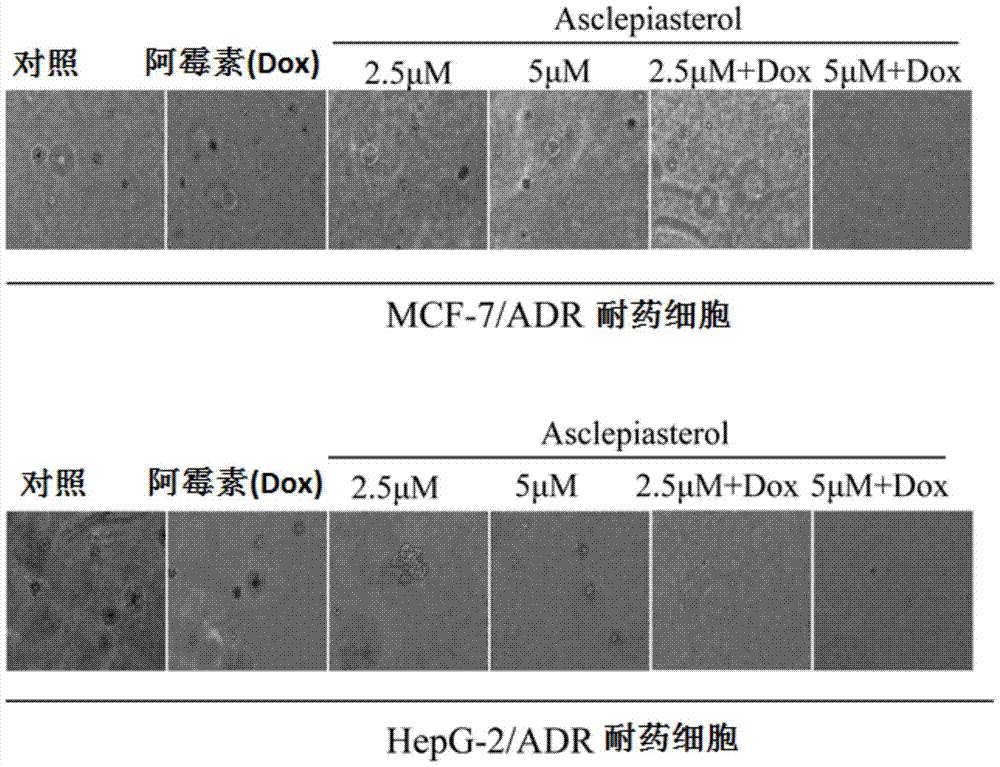
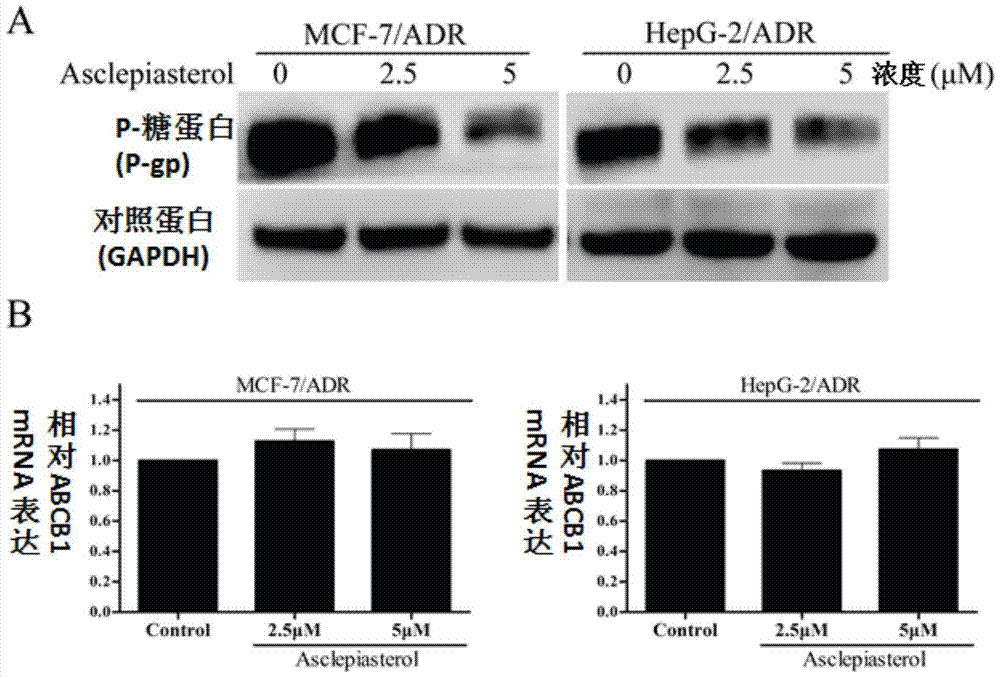
C21 steroid asclepiasterol and its preparation method and its application in the preparation of tumor multidrug resistance reversal agent
A multi-drug resistance, tumor technology, applied in the field of natural medicinal chemistry, to achieve the effect of good tumor multi-drug resistance reversal activity, rich content, and increased accumulation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]Extract 1 kg of the whole milkweed herb twice with 3 times the amount of 50% alcohol, combine the extracts, and concentrate under reduced pressure to obtain the total extract (95 g). Dilute the total extract with water to obtain a diluent; add cyclohexane to the diluent for extraction, and collect the extracted cyclohexane layer; then add ethyl acetate to the remaining water layer for extraction, and collect the ethyl acetate layer, the aqueous layer was discarded, and the ethyl acetate layer was concentrated under reduced pressure at 30° C. to obtain the ethyl acetate fraction (16 g) of the milkweed total extract. The ethyl acetate part was chromatographed on a silica gel (200 mesh, 160g) column, using CHCl with a volume ratio of 100:0, 100:1, 50:1, 20:1, and 10:1 in sequence 3 -MeOH was eluted with two column volumes each. The 10:1 eluted fraction was recrystallized from methanol to give the C21 steroid Asclepiasterol (52 mg).
Embodiment 2
[0038] 1 kg of the whole milkweed herb was extracted 4 times with 5 times the amount of 100% ethanol, and the extracts were combined and concentrated under reduced pressure to obtain the total extract (95 g). Dilute the total extract with water to obtain a diluent; add cyclohexane to the diluent for extraction, and collect the extracted cyclohexane layer; then add ethyl acetate to the remaining water layer for extraction, and collect the ethyl acetate layer, the aqueous layer was discarded, and the ethyl acetate layer was concentrated under reduced pressure at 50° C. to obtain the ethyl acetate fraction (16 g) of the milkweed total extract. The ethyl acetate part was chromatographed on a silica gel (300 mesh, 160g) column, using CHCl with a volume ratio of 100:0, 100:1, 50:1, 20:1, and 10:1 in sequence 3 -MeOH was eluted with two column volumes each. The 10:1 eluted fraction was recrystallized from methanol to give the C21 steroid Asclepiasterol (63 mg).
Embodiment 3
[0040] 1 kg of whole milkweed was extracted 3 times with 4 times the amount of 70% alcohol, the combined extracts were concentrated under reduced pressure to obtain the total extract (100.5 g). Dilute the total extract with water to obtain a diluent; add cyclohexane to the diluent for extraction, and collect the extracted cyclohexane layer; then add ethyl acetate to the remaining water layer for extraction, and collect the ethyl acetate layer, the aqueous layer was discarded, and the ethyl acetate layer was concentrated under reduced pressure at 40° C. to obtain the ethyl acetate fraction (16 g) of the milkweed total extract. The ethyl acetate part was chromatographed on a silica gel (250 mesh, 160g) column, using CHCl with a volume ratio of 100:0, 100:1, 50:1, 20:1, and 10:1 in sequence 3 -MeOH was eluted with two column volumes each. The 10:1 eluted fraction was recrystallized from methanol to give the C21 steroid Asclepiasterol (58 mg).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com