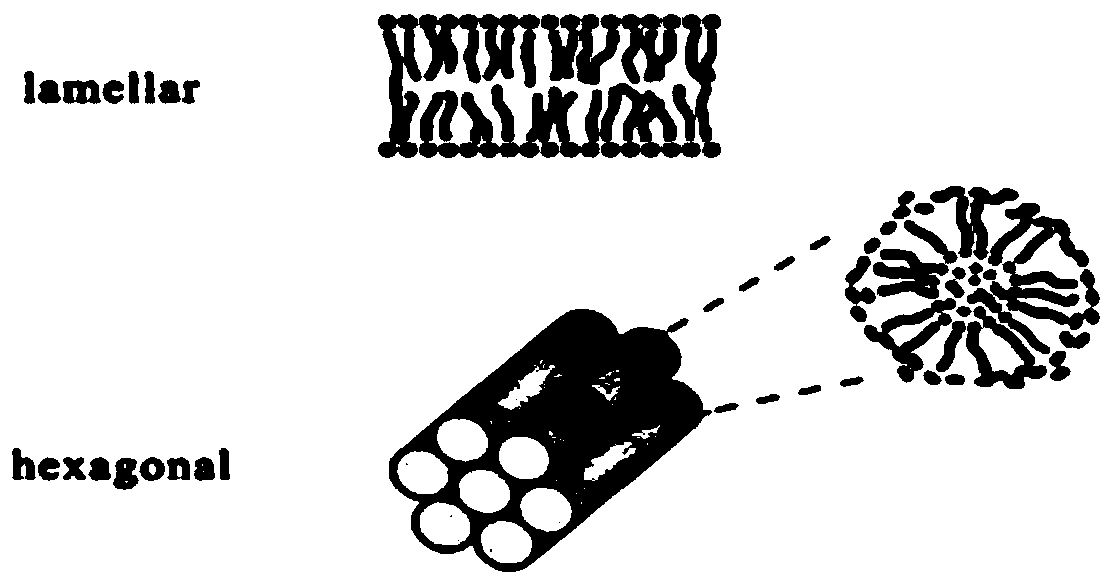
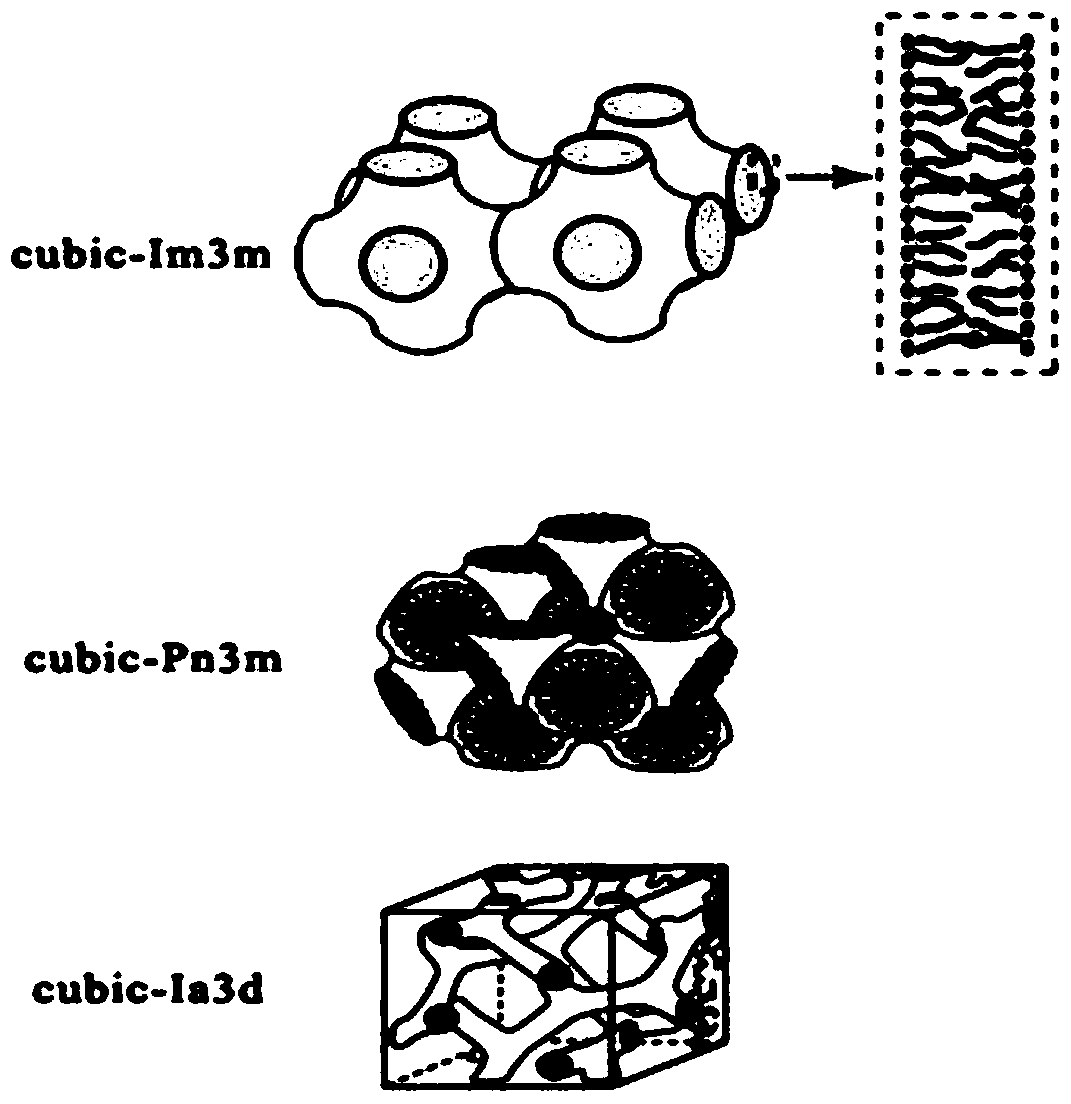
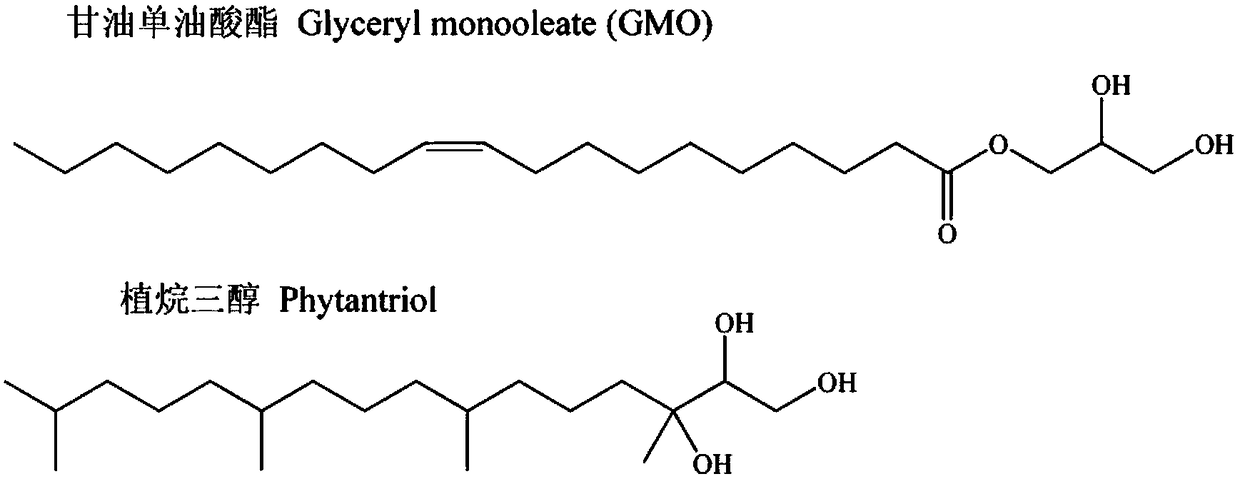
Lyotropic liquid crystal precursor for root canal disinfection and its preparation method and application
A lyotropic liquid crystal and root canal technology, applied in the field of lyotropic liquid crystal precursors and their preparation, can solve the problems of no application of lyotropic liquid crystal precursors, reduced root canal treatment effect, inability to perform disinfection and sterilization, etc. The effect of releasing long-term root canal sealing, eliminating the blind spot of disinfection, and important practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1 Preparation and viscosity test of chlorhexidine acetate-nanometer silver-glycerol monooleate lyotropic liquid crystal precursor
[0071] The mass ratio of glycerol monooleate-absolute ethanol-water is an isotropic solution of 12:3:10 as a drug carrier, and chlorhexidine acetate and nano silver are used as medicines for root canal disinfection; The lyotropic liquid crystal precursor for root canal disinfection, its preparation method comprises the following steps:
[0072] Weigh 0.6g of absolute ethanol, add 0.01g, 0.025g and 0.05g of chlorhexidine acetate respectively (the mass fraction of the last gained lyotropic liquid crystal precursor is respectively 0.2%, 0.5% and 1%), then add 2.4 g melted glycerol monooleate at 42°C, mixed evenly, and then added 2 g of finished nano-silver aqueous solutions diluted with water (which contained 0.001 g and 0.0005 g of nano-silver respectively, that is, in the final obtained lyotropic liquid crystal precursor) The mas...
Embodiment 2
[0076] Example 2 In vitro release test of chlorhexidine acetate-nano silver-glycerol monooleate lyotropic liquid crystal precursor
[0077] The in vitro release characteristics of chlorhexidine acetate and nano-silver in lyotropic liquid crystal precursor were investigated by membraneless dissolution method. 0.2g of lyotropic liquid crystal precursor (preparation method is the same as in Example 1, with different contents of chlorhexidine acetate and nano-silver) was dropped into 30ml of deionized water, and simulated in vitro release in a shaker at 37°C and 100rpm, Take 2ml samples at intervals and store them at 4°C for testing, then add an equal amount of release medium to continue simulating in vitro release under the above conditions, and after 30 days, measure chlorhexidine acetate and nano-silver in the lyotropic liquid crystal precursor with a UV spectrophotometer of drug release. The result is as Figure 4 and Figure 5 shown. It can be seen from the results that t...
Embodiment 3
[0079] Example 3 Effects of different adding methods of nano-silver on the physical stability of lyotropic liquid crystal precursors
[0080] According to the preparation method of the lyotropic liquid crystal precursor in Example 1, a lyotropic liquid crystal precursor containing 0.5% chlorhexidine acetate compounded with 0.02% nano-silver was prepared as an experimental group. And the preparation method of the lyotropic liquid crystal precursor of control group comprises the following steps:
[0081] Weigh 0.6g of absolute ethanol, add 0.025g of chlorhexidine acetate, then add 2.4g of glycerol monooleate melted at 42°C, mix well, then add 0.001g of nano-silver powder, and finally add 2g of water dropwise, vortex Spinning and mixing, after centrifugation, sealed and placed at room temperature for one week, the lyotropic liquid crystal precursor compounded with 0.5% chlorhexidine acetate of the control group and 0.02% nano-silver was obtained.
[0082] After standing for a we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com