Patents
Literature
281results about "Filtration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
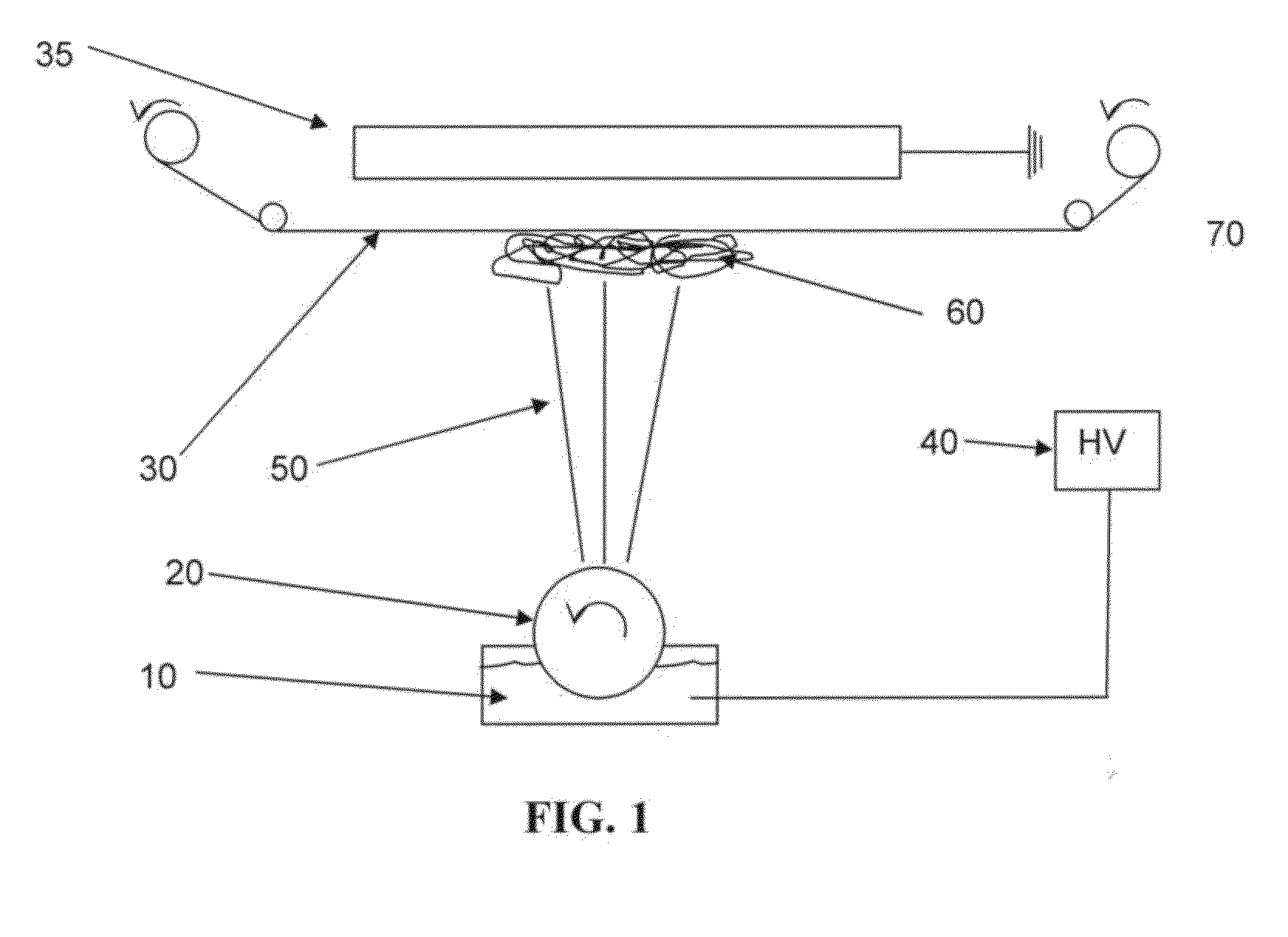
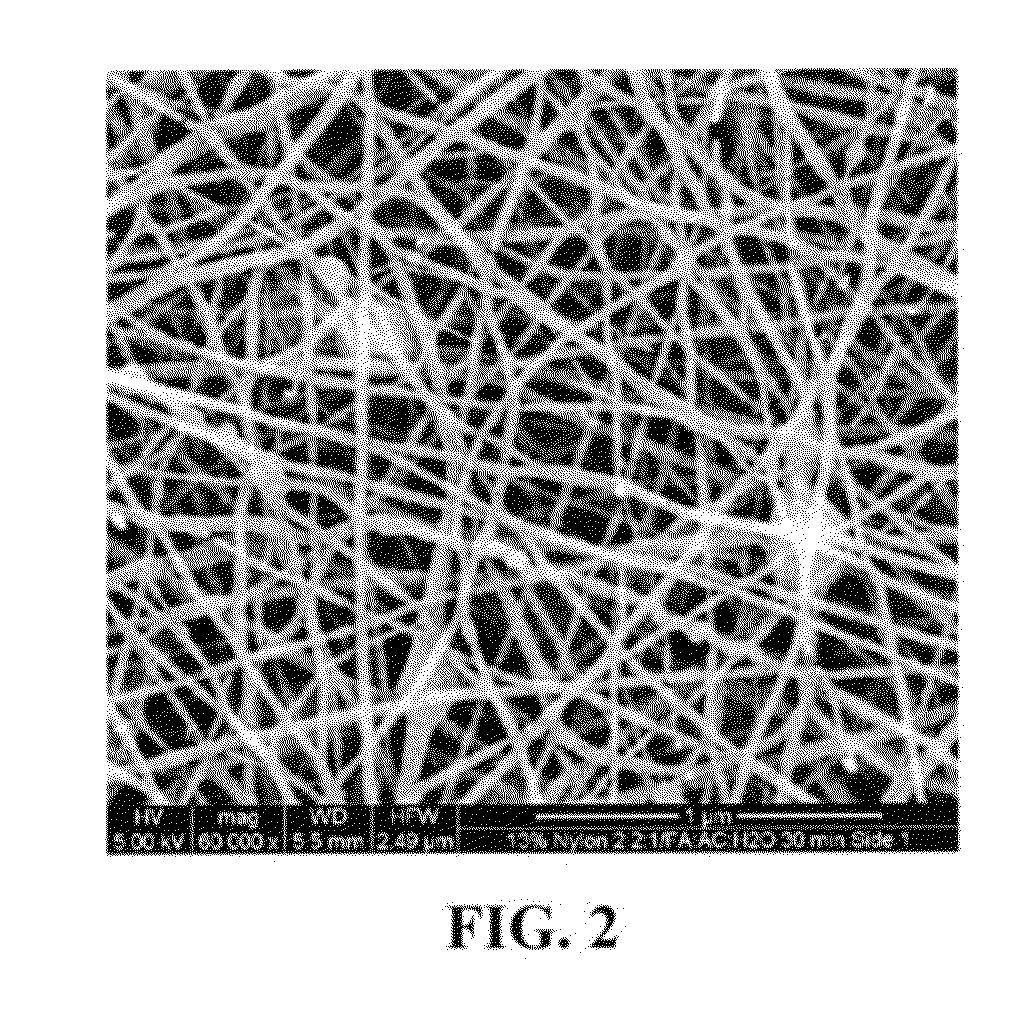
Nanosize electropositive fibrous adsorbent
InactiveUS6838005B2Improve the immunityEfficient precipitationIon-exchanger regenerationLoose filtering material filtersFiberParticulates
Aluminum hydroxide fibers approximately 2 nanometers in diameter and with surface areas ranging from 200 to 650 m2 / g have been fount to be highly electropositive. When dispersed in water they are able to attach to and retain electronegative particles. When combined into a composite filter with other fibers or particles they can filter bacteria and nano size particulates such as viruses and colloidal particles at high flux through the filter. Such filters can be used for purification and sterilization of water, biological, medical and pharmaceutical fluids, and as a collector / concentrator for detection and assay of mirobes and viruses. The alumina fibers are also capable of filtering sub-micron inorganic and metallic particles to produce ultra pure water. The fibers are suitable as a substrate for growth of cells. Macromolicules such as proteins may be separated from each other based on their electronegative charges.
Owner:ARGONIDE CORP
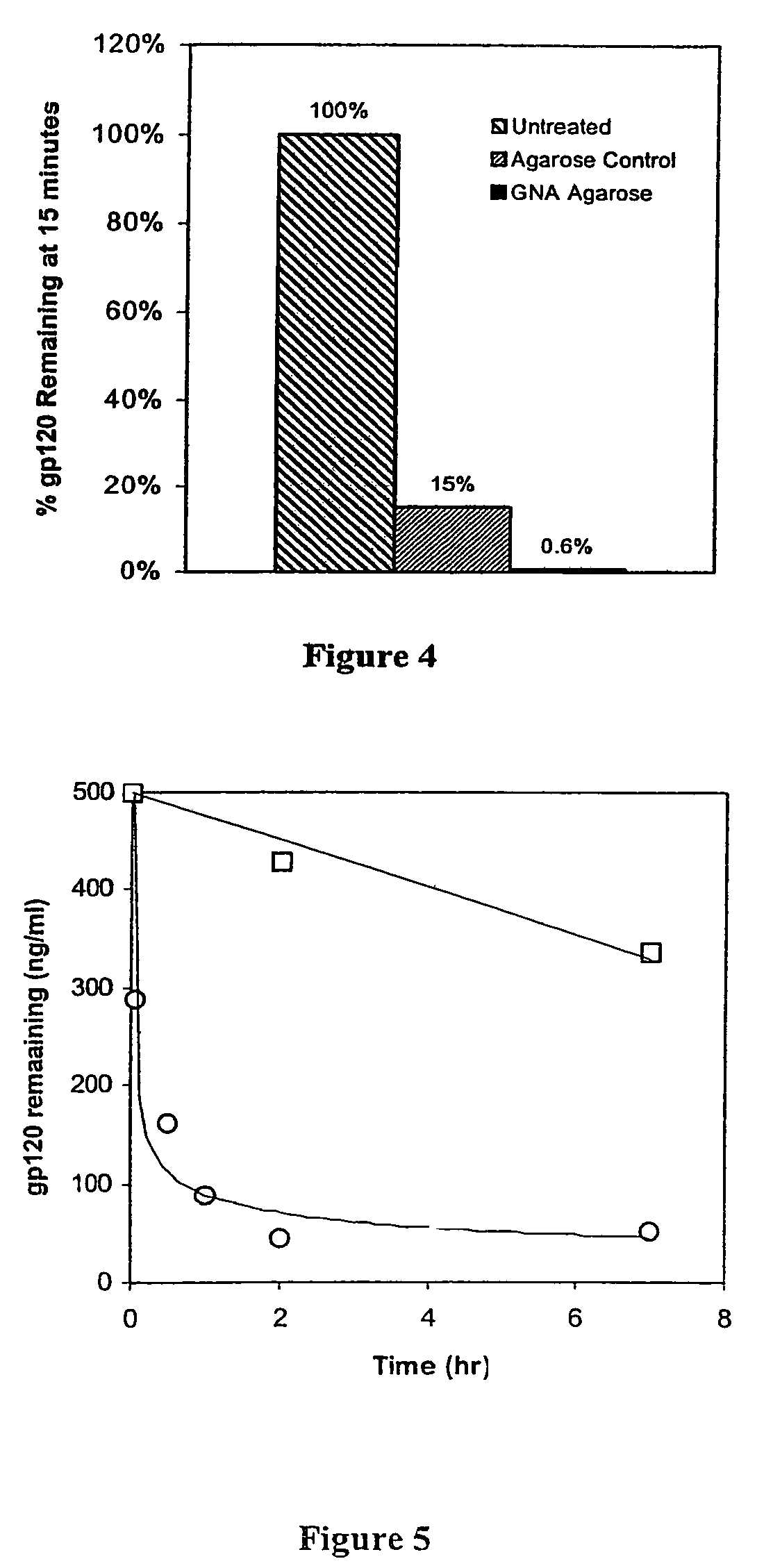
Methods for removal, purification, and concentration of viruses, and methods of therapy based thereupon
InactiveUS6861001B2Effect clotting timeEasy to zoom inBioreactor/fermenter combinationsBiological substance pretreatmentsSide chainBlood plasma
The invention is based on the discovery that certain membranes, which include side chains or molecular “brushes” having, for example, tertiary amino functional groups, can be used as highly effective filters to capture viruses / virus particles from liquids without removal of proteins. New methods based on this discovery include removing viruses from liquids such as blood or plasma, removing viruses from pharmaceuticals, concentrating and / or purifying viruses, e.g., for use in gene therapy, and producing recombinant viruses in new bioreactors. The invention also includes new methods of therapy or adjunct therapy for viral infections, in which a patient's blood or plasma is filtered through the membranes to remove viruses to reduce the viral load. The invention also includes new bioreactors and viral filters containing the membranes.
Owner:THE GENERAL HOSPITAL CORP
Pathogen inactivating compositions for disinfecting biological fluids
InactiveUS6106773AAvoid bulk processingAvoid capital intensive equipmentBiocideSolvent extractionBlood componentAqueous solution
The present invention is directed to pathogen inactivating compositions that can be used to disinfect various biological fluids, such as blood, blood fractions, and the like. The compositions are suitable for disinfecting biological fluids containing valuable, but labile, components such as proteins without destroying the desired properties of such components. The pathogen inactivating compositions of the present invention are produced by contacting water or an aqueous solution with iodinated matrix material. The compositions can be pre-formulated and stored for subsequent use in disinfecting a wide range of biological fluids.
Owner:AMERICAN NAT RED CROSS
Last-chance quality check and/or air/pathogen filtger for infusion systems
ActiveUS20080203023A1Small apertureIncrease pressure dropSolvent extractionHaemofiltrationBlood treatmentsHigh rate
Blood treatment system and method for high rate hemofiltration ensures against pyrogenic patient reaction by providing various mechanisms for filtering replacement fluid to remove endotoxins and other safety features including detecting incorrect fluid administration.
Owner:NXSTAGE MEDICAL INC
Sterilization of Biosensors
InactiveUS20070111196A1Microbiological testing/measurementDiagnostic recording/measuringBinding domainEnzyme
The present invention relates to methods of making a sterilized biosensor, where the biosensor comprises at least one binding reagent, which comprises at least one non-enzyme proteinaceous binding domain. Certain embodiments of the methods described herein comprise partially assembling the components of the biosensor, except for the binding reagent, and separately sterilizing this partial assemblage and the binding reagent. The sterilized binding reagent and the sterilized partial assemblage are then aseptically assembled to produce the sterilized biosensor. Other embodiments of the methods described herein comprise assembling substantially all of the components of the biosensor, including the binding reagent, and sterilizing the assembled biosensor to produce a sterilized biosensor.
Owner:BECTON DICKINSON & CO
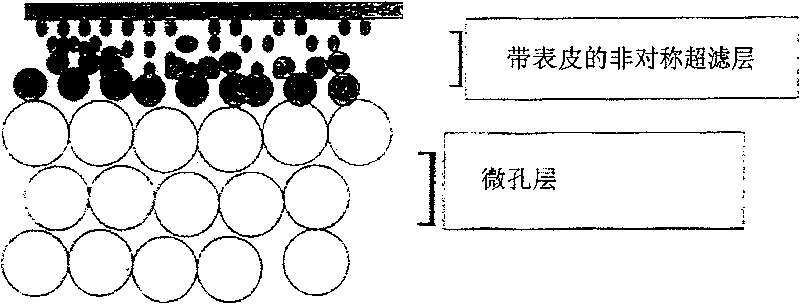
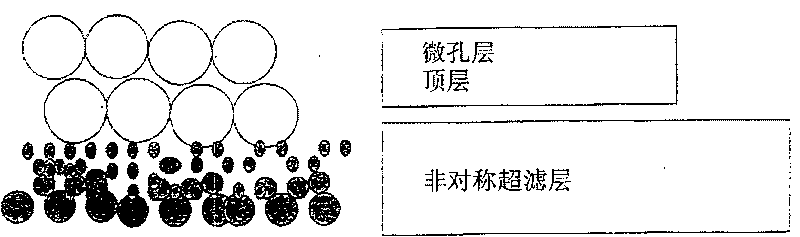
Microporous filter media, filteration systems containing same, and methods of making and using
InactiveUS6913154B2Material nanotechnologySpace heating and ventilationNatural organic matterFilter media
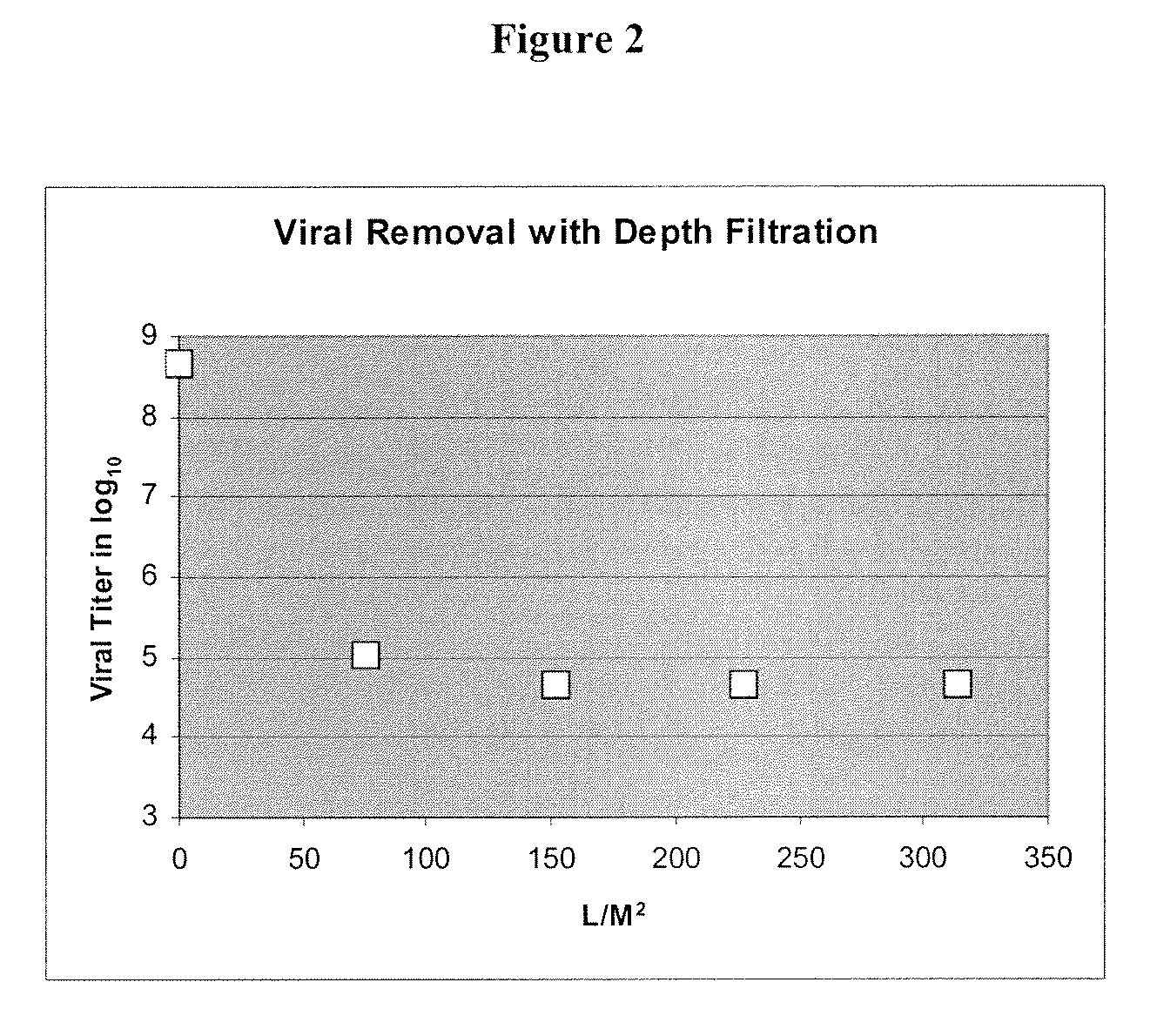
The invention is directed to a microbiological interception enhanced filter medium, preferably having an adsorbent prefilter located upstream from the filter medium. Preferably, the prefilter is adapted to remove natural organic matter in an influent prior to the influent contacting the microbiological interception enhanced filter medium, thereby preventing loss of charge on the filter medium. The microbiological interception enhanced filter medium is most preferably comprised of fibrillated cellulose fibers, in particular, lyocell fibers. At least a portion of the surface of the at least some of the fibers have formed thereon a microbiological interception enhancing agent comprising a cationic metal complex. A filter medium of the present invention provides greater than about 4 log viral interception, and greater than about 6 log bacterial interception.
Owner:KX TECH LLC (DW US)
Device and method for reducing inflammatory mediators in blood
InactiveUS7201730B2Reducing free radicals in a patient's bloodReduce concentrationSemi-permeable membranesSolvent extractionInterleukin 6Staphylococcus cohnii
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50–75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
Method of making an antimicrobial sintered porous plastic filter
InactiveUS6849214B2More hygienic conditionReduce concentrationUltrafiltrationTreatment involving filtrationThermoplastic
An improved sintered porous thermoplastic filter incorporating a second thermoplastic treated with a non-leaching antimicrobial agent for the purification of liquids. The method of making the filter is also disclosed.
Owner:MICROBAN PROD CO INC
Microporous filter media, filtration systems containing same, and methods of making and using
InactiveUS6953604B2Space heating and ventilationTreatment involving filtrationNatural organic matterFilter system
The invention is directed to a microbiological interception enhanced filter medium, preferably having an adsorbent prefilter located upstream from the filter medium. Preferably, the prefilter is adapted to remove natural organic matter in an influent prior to the influent contacting the microbiological interception enhanced filter medium, thereby preventing loss of charge on the filter medium. The microbiological interception enhanced filter medium is most preferably comprised of fibrillated cellulose fibers, in particular, lyocell fibers. At least a portion of the surface of the at least some of the fibers have formed thereon a microbiological interception enhancing agent comprising a cationic metal complex. A filter medium of the present invention provides greater than about 4 log viral interception, and greater than about 6 log bacterial interception.
Owner:KX TECH LLC (DW US)
Method for removal of viruses from blood by lectin affinity hemodialysis
ActiveUS7226429B2Reduce loadReduce in quantityOther blood circulation devicesViral antigen ingredientsHemodialysisHigh mannose
The present invention relates to a method for using lectins that bind to pathogens having high mannose surface glycoproteins or fragments thereof which contain high mannose glycoproteins, to remove them from infected blood or plasma in an extracorporeal setting. Accordingly, the present invention provides a method for reducing viral load in an individual comprising the steps of obtaining blood or plasma from the individual, passing the blood or plasma through a porous hollow fiber membrane wherein lectin molecules are immobilized within the porous exterior portion of the membrane, collecting pass-through blood or plasma and reinfusing the pass-through blood or plasma into the individual.
Owner:AETHLON MEDICAL INC
Removal of microorganisms from fluid samples using nanofiber filtration media
A method for removing microorganisms from liquid samples and a nanofiber containing liquid filtration medium that simultaneously exhibits high liquid permeability and high microorganism retention. Microorganisms such as bacteria, particularly B. Diminuta, are removed from a liquid by passing the liquid through a porous nanofiber containing filtration medium having a B. Diminuta LRV greater than about 9, and the nanofiber(s) has a diameter from about 10 nm to about 1,000 nm. Another method for removing microorganisms such as bacteria and Mycloplasma, includes passing the liquid through a porous nanofiber containing filtration medium having a microorganism LRV greater than about 8, and the nanofiber(s) has a diameter from about 10 nm to about 1,000 nm. The filtration medium can be in the form of a fibrous electro spun polymeric nanofiber liquid filtration medium mat.
Owner:MILLIPORE CORP
Charged membrane
InactiveUS6849185B1Ion-exchanger regenerationSolid sorbent liquid separationBacterial contaminantsMicroporous membranes
The present invention provides a hydrophilic charged microporous membrane comprising a porous hydrophobic substrate and a coating comprising a charge-providing agent. The present invention further provides a hydrophilic charged membrane comprising a porous hydrophobic substrate and a hydrophilic charge-providing agent distributed within the substrate. The present invention further provides a filter as well as a filter device comprising the inventive hydrophilic charged membrane. The present invention further provides a process for treating a fluid containing bacterial contaminants such as endotoxins comprising contacting the membrane with the fluid to provide a bacterial contaminant depleted fluid.
Owner:PALL CORP
Method for the validatable inactivation of pathogens in a biological fluid by irradiation
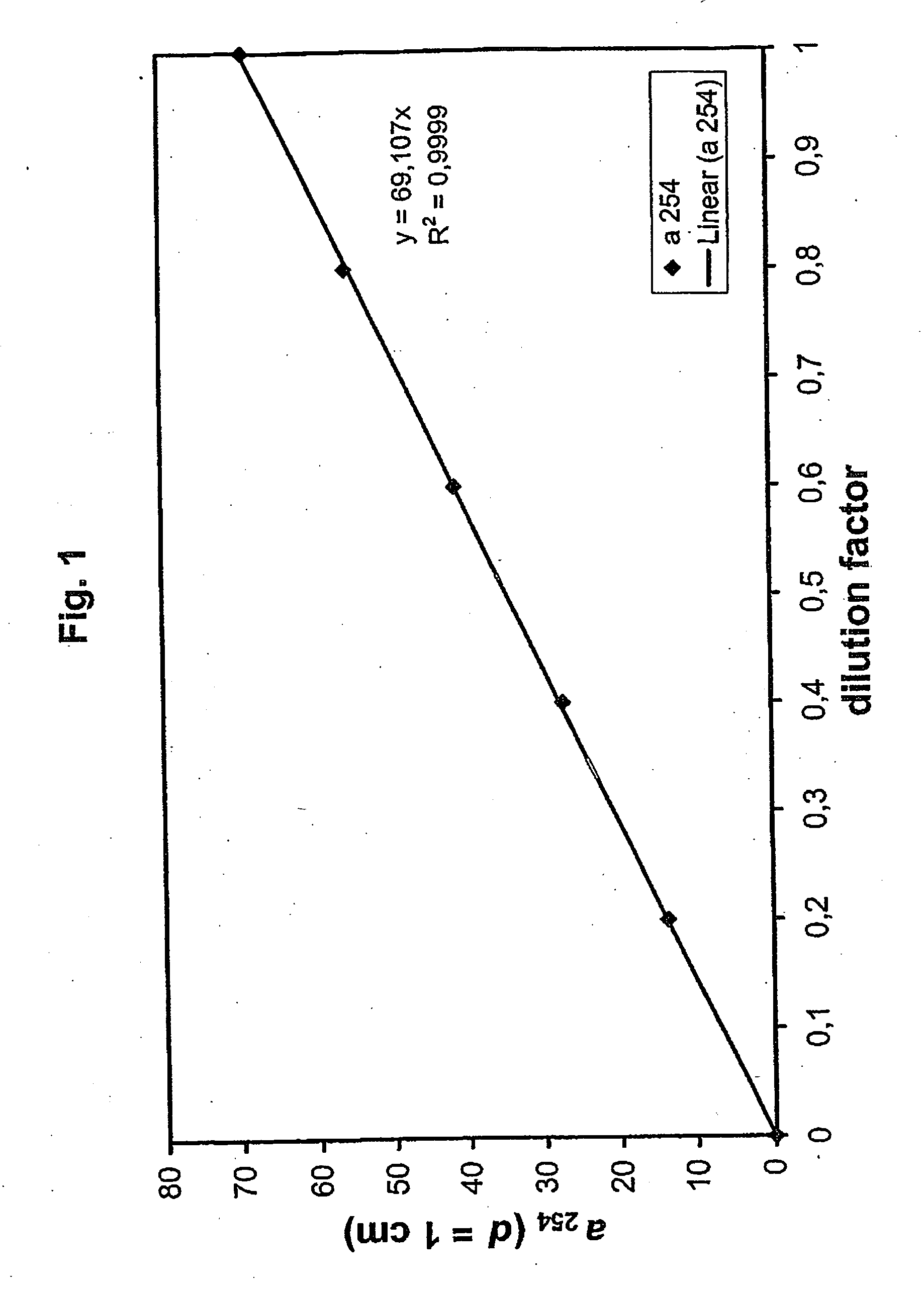
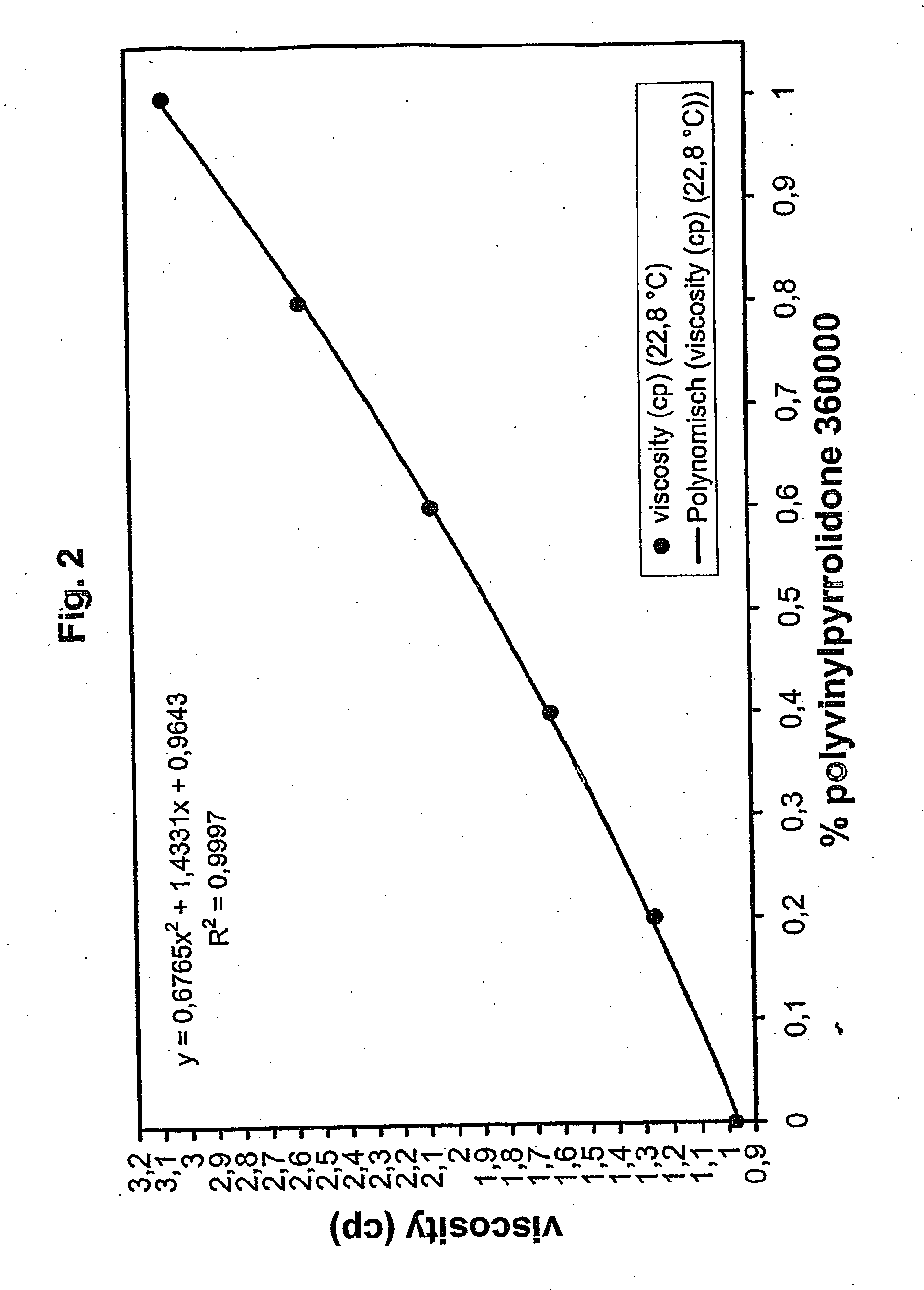
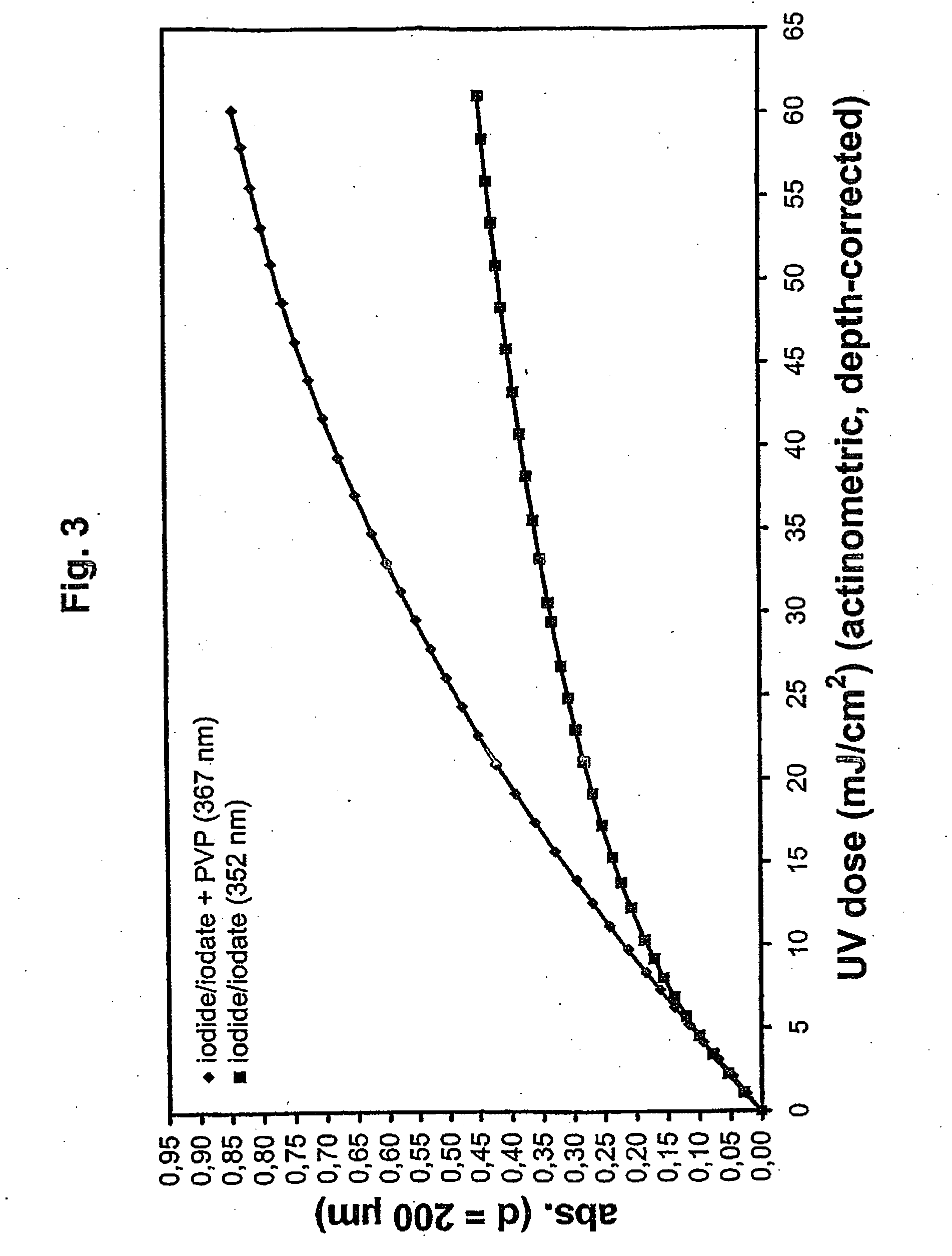
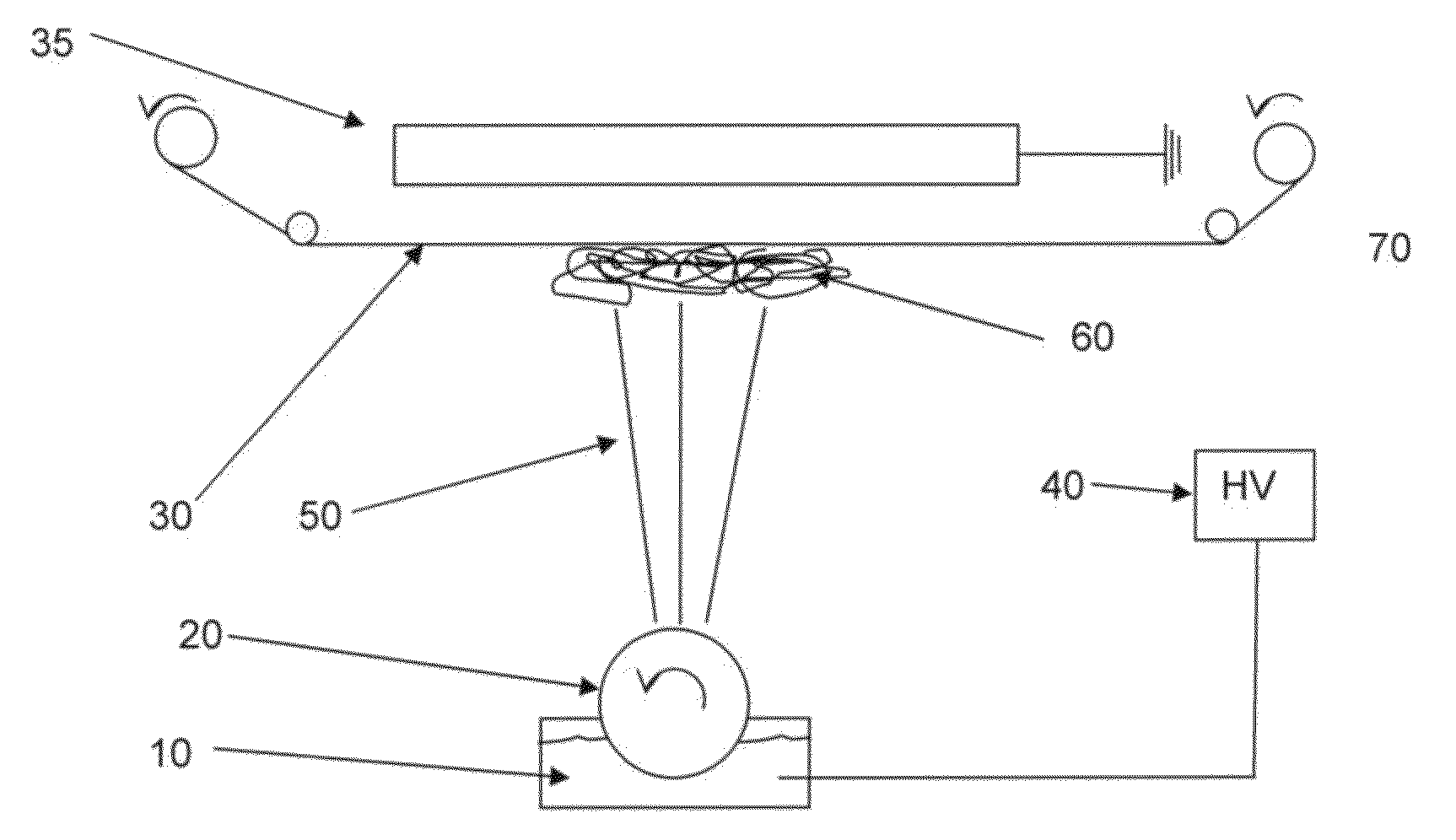
ActiveUS20060257877A1Avoid damageReduce lossWater/sewage treatment by irradiationMicrobiological testing/measurementValidation methodsBiochemistry
A validatable method for determining a photochemically effective dose for inactivating pathogens in a fluid sample is described herein. In particular, the instant invention covers methods for determining a photochemically effective dose sufficient to inactivate pathogens in a biological sample while leaving biologically active substances of interest unaffected. A batch irradiation reactor effective for inactivating pathogens in biological samples is also described.
Owner:TAKEDA PHARMA CO LTD
Method for retrovirus removal
A method for removing retroviruses from liquid samples and a nanofiber containing liquid filtration medium that simultaneously exhibits high liquid permeability and high microorganism retention is disclosed. Retroviruses are removed from a liquid by passing the liquid through a porous nanofiber containing filtration medium having a retrovirus LRV greater than about 6, and the nanofiber(s) has a diameter from about 10 nm to about 100 nm. The filtration medium can be in the form of a fibrous electrospun polymeric nanofiber liquid filtration medium mat.
Owner:MILLIPORE CORP
Vacuum cleaner attachment for fungi removal and method of use thereof
InactiveUS20040020007A1Good removal effectClean fungal sporeSuction nozzlesLavatory sanitoryHEPAParticulates
A vacuum cleaner attachment and method of use thereof provides an effective cleaning system for fungi and the like. A vacuum paddle extension enables the attachment to travel into narrow cracks and crevices where fungus may grow and by use of an abrasive and porous pad dislodges such fungus or fungi. When attached to a vacuum system, such as a HEPA (High Efficiency Particulate Air-Filtered) vacuum system, the debris and particulate matter generated by the abrasive process is drawn into the vacuum attachment and into the vacuum system where it is removed from the air which is then expelled by the vacuum system. Better cleaning of surfaces afflicted with fungi, molds, and the like is then achieved in a manner that is safer and more effective.
Owner:LAUSEVIC JOHN
Ultrafiltration membranes and methods of making and use of ultrafiltration membranes
InactiveUS20070084788A1Minimizing flow restrictionUniform layersMembranesSemi-permeable membranesCelluloseProtein solution
An inherently hydrophobic polymer membrane substrate having its surface rendered hydrophilic with a hydroxyalkyl cellulose and having a throughput greater than about 1500 L / m2 is provided. A process for making the membrane also is provided which includes a step of autoclaving in boiling water and / or steam or submerging in boiling water. The membrane is useful for removing virus from a protein solution.
Owner:MILLIPORE CORP
Adsorptive membranes for trapping viruses
ActiveUS20080014625A1Release resourcesImprove usabilityMembranesSemi-permeable membranesAdsorptive membraneTrapping
A disposable, virus-trapping membrane, and a corresponding method to remove viruses from solution are described. The membrane includes a disposable, micro-porous filter membrane and a ligand immobilized on the membrane. The ligand irreversibly and selectively binds viruses. The ligand also has a pKa sufficiently high to repel antibodies via electrostatic charge repulsion.
Owner:WISCONSIN ALUMNI RES FOUND
Filter assembly for a reprocessor
InactiveUS7135142B2Reduce Microbial ContaminationReduce the possibilityDetection of fluid at leakage pointTreatment involving filtrationWater useReprocessor
Owner:AMERICAN STERILIZER CO
Blood treatment device and method with selective solute extraction
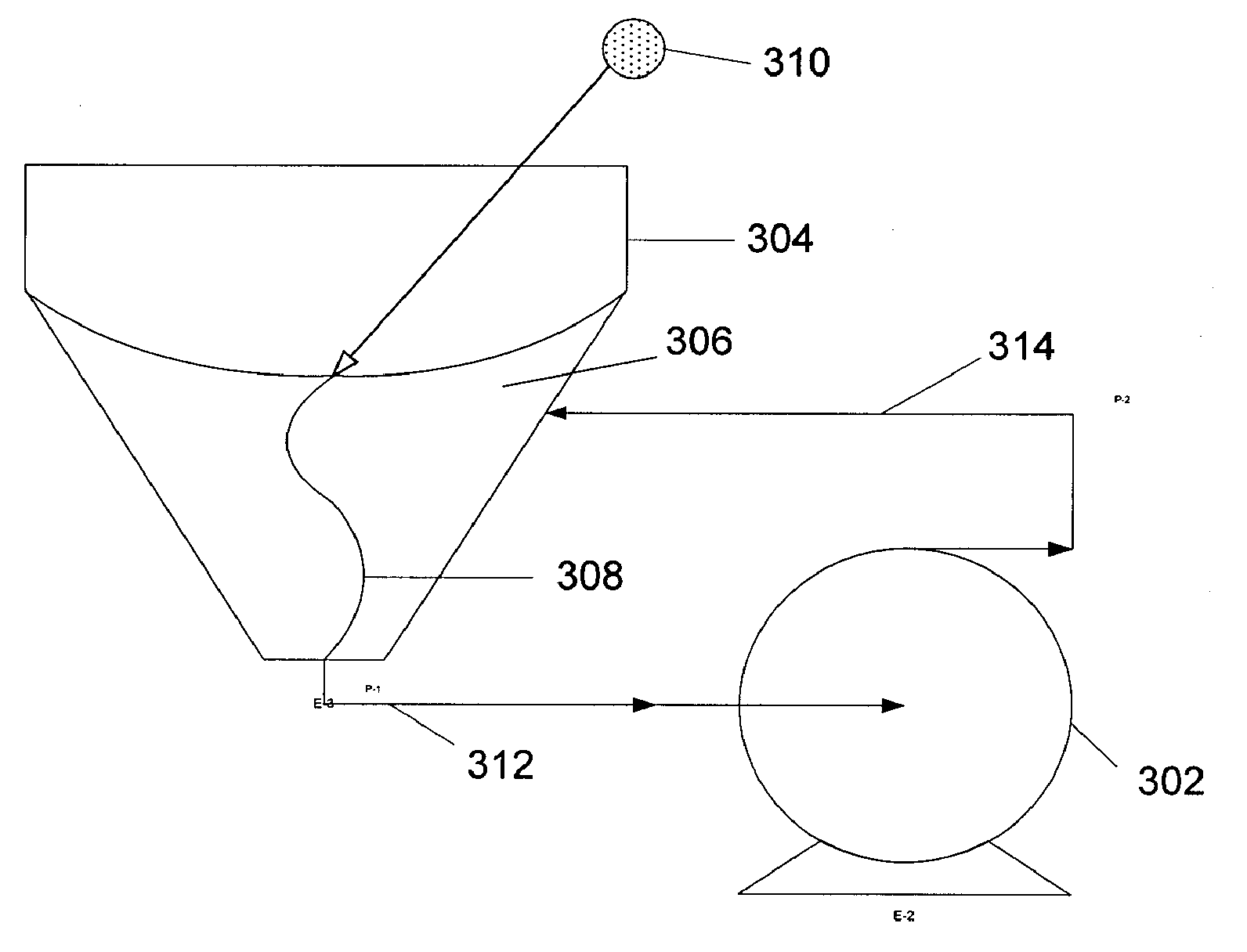
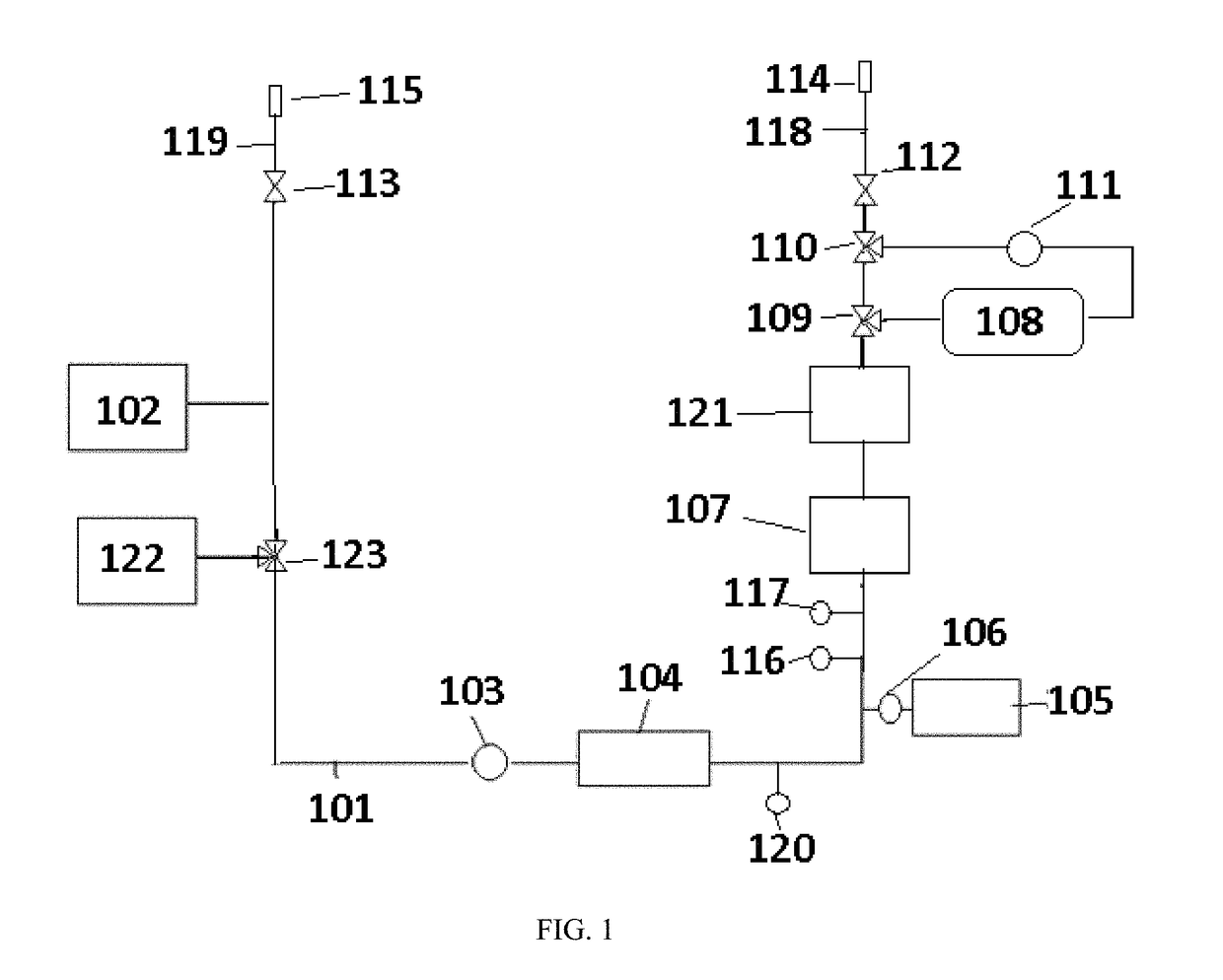
The object of the present invention is the filtration of blood in order to selectively separate and extract molecules of a given size via extracorporeal systems for separating substances. The invention thus concerns an extracorporeal blood treatment device including: one or more exchangers (1) comprising a first inlet (2) for the blood to be treated, a first fluid outlet (4) and a second fluid outlet (5), an inlet line (10) for the blood to be treated, connected to the first inlet (2) of the exchanger (1), a blood outlet line (or venous line) (11) coupled to the first outlet (4) of the exchanger (1), one or more treatment units (21) comprising at least a first fluid inlet (22) and at least a first fluid outlet (24), wherein the second outlet (5) of the exchanger (1) is in fluid communication with the first inlet (22) of the treatment unit (21), the invention being characterised in that the first outlet (24) of the treatment unit (21) is in fluid communication with the inlet line (10).
Owner:GAMBRO LUNDIA AB
Methods for Removing Viral Contaminants During Protein Purification
The present invention relates, in general, to methods for removing viral contaminants from therapeutic protein solutions to improve safety of therapeutic proteins administered to patients. Particularly contemplated is the removal of small non-enveloped viruses, such as parvovirus, from therapeutic protein solutions.
Owner:AMGEN INC +1
Air decontamination unit
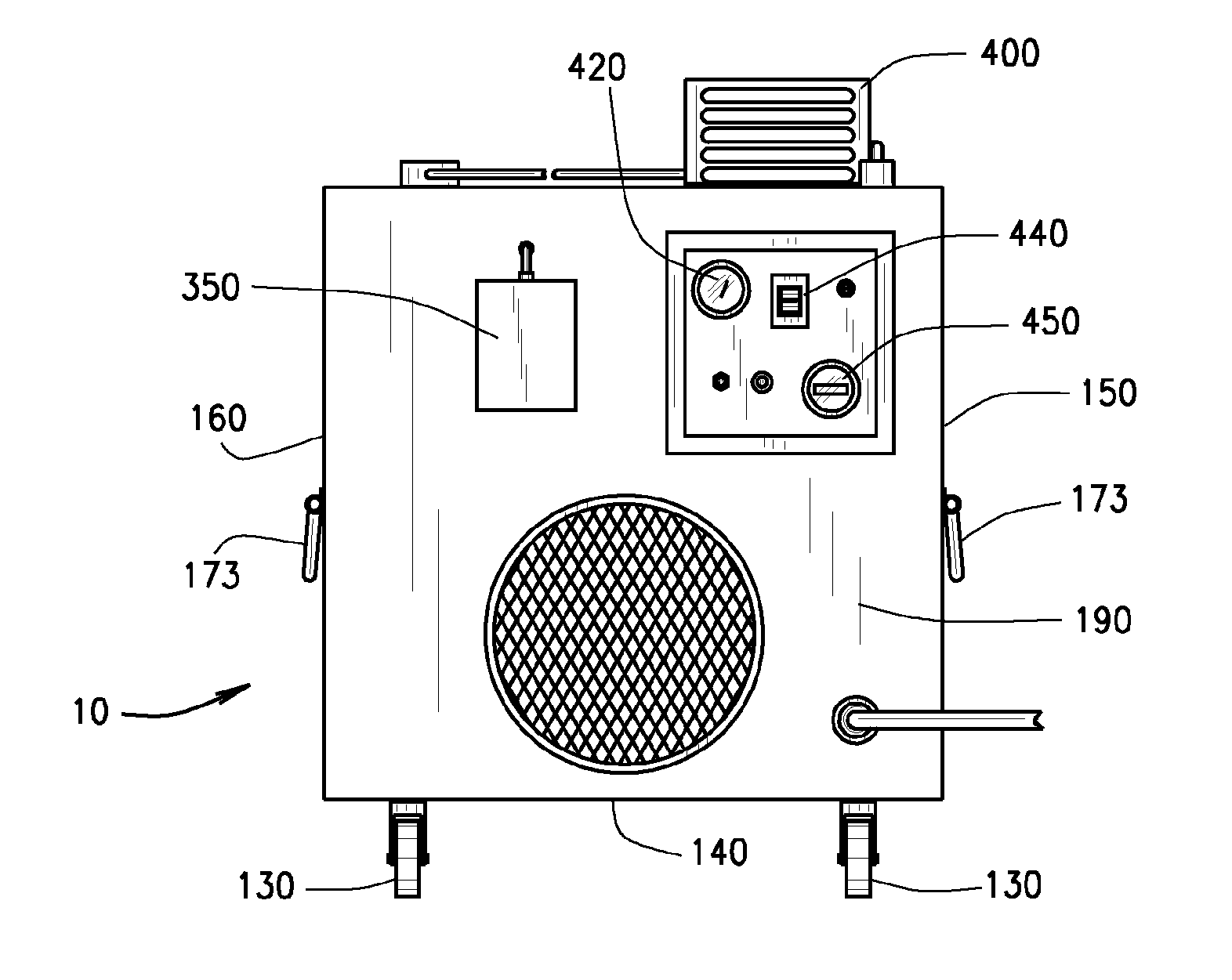
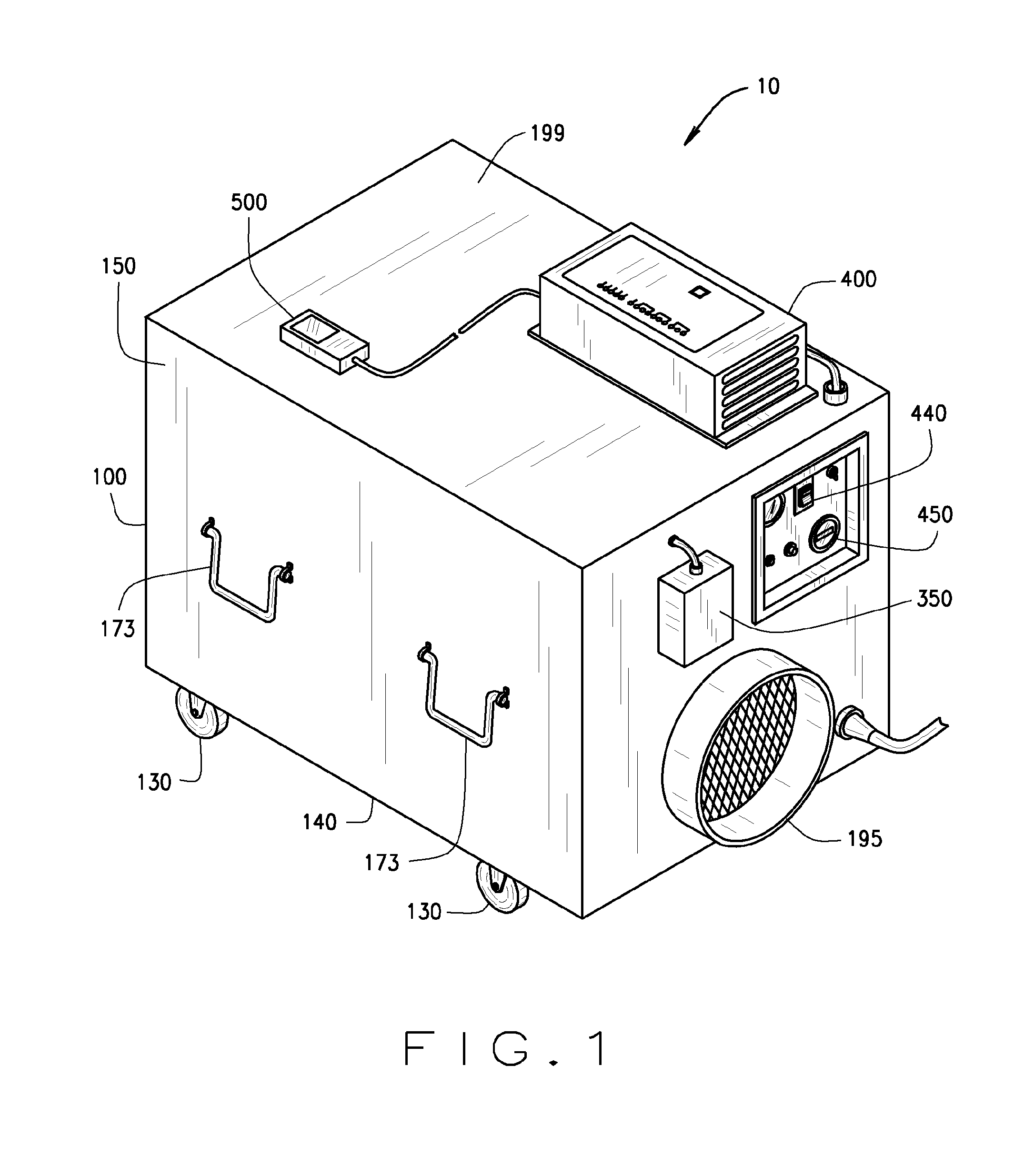
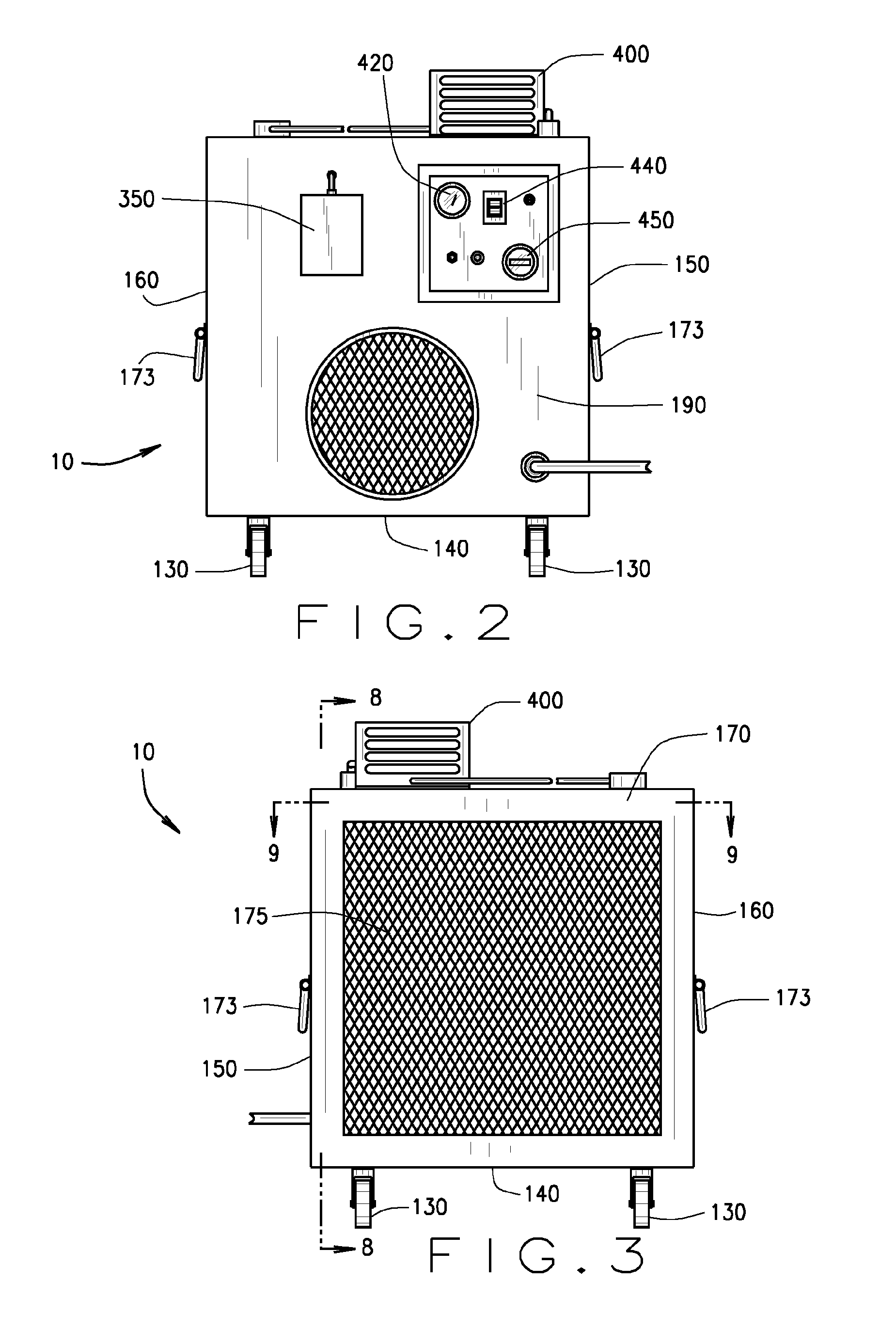
InactiveUS20110165018A1Easy to moveIncrease speedCombination devicesCosmetic preparationsEnvironmental engineeringDirect communication
An air decontamination unit is described with a housing defining an interior, an intake and exhaust in direct communication with the interior, a blower—in operational communication with a control panel—for drawing in and moving air through the housing, which further includes a filter assembly, a UV lamp, an ozone lamp, and an ozone sensor for detecting the concentration of ozone therein—positioned in the interior of the housing, all in direct communication with the control panel.
Owner:CLEANCORE SOLUTIONS LLC
Ultraporous Nanofiber Mats And Uses Thereof
A porous electrospun polymeric nanofiber liquid filtration medium, such as an electrospun mats, used for the removal of viral particles (e.g., parvovirus) and other particles in the 18 nm to 30 nm size range from fluid streams, having a mean flow bubble point measured with perfluorohexane above 100 psi. The electrospun medium includes nanofibers having an average fiber diameter of about 6 nm to about 13 nm, and the nanofiber liquid filtration medium has a mean pore size ranging from about 0.01 um to about 0.03 um, a porosity ranging from about 80% to about 95%, a thickness ranging from about 1 um to about 100 um, and a liquid permeability greater than about 10 LMH / psi. The high porosity of the electro-spun mats enable much higher water fluxes, thus reducing the time required to complete virus filtration steps on a fluid stream.
Owner:MILLIPORE CORP
Ultrafiltration membrane and preparation method thereof
The present invention provides a multi-layer ultrafiltration composite membrane which has at least one ultrafiltration layer made by cocoating or sequentially coating a high purity polymer solution onto a support to form a raw membrane sheet, and then the membrane sheet is immerged in coagulation liquid to effect phase separation, thus, a multilayered composite membrane contains at least one ultrafiltration layer can be obtained.
Owner:MILLIPORE CORP
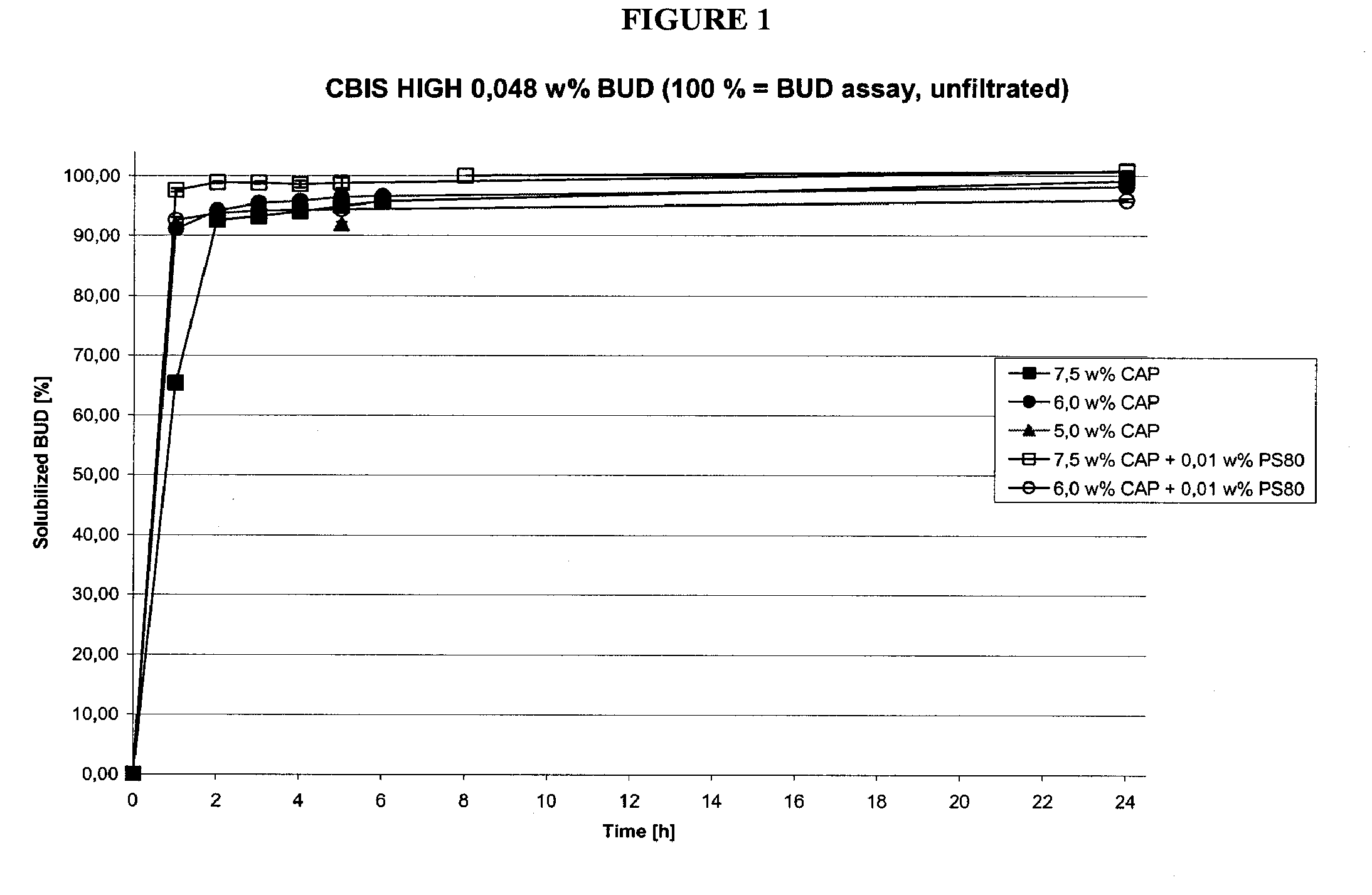
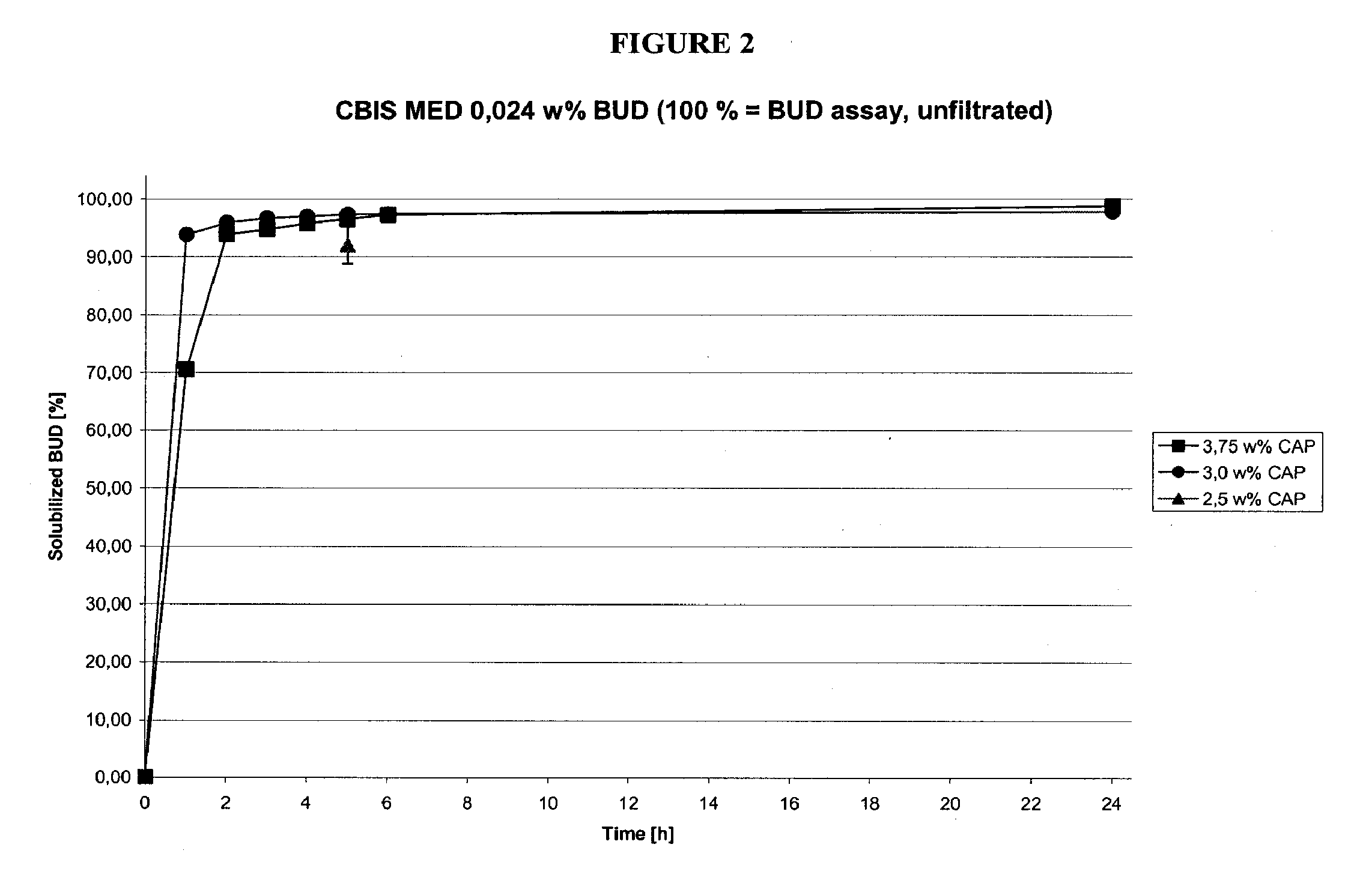
Methods of manufacturing cortiscosteroid solutions
The present invention relates to methods of manufacturing compositions comprising a corticosteriod and at least one solubility enhancer, as well as compositions made by these methods.
Owner:TIKA LAEKEMEDEL AB
Isolator
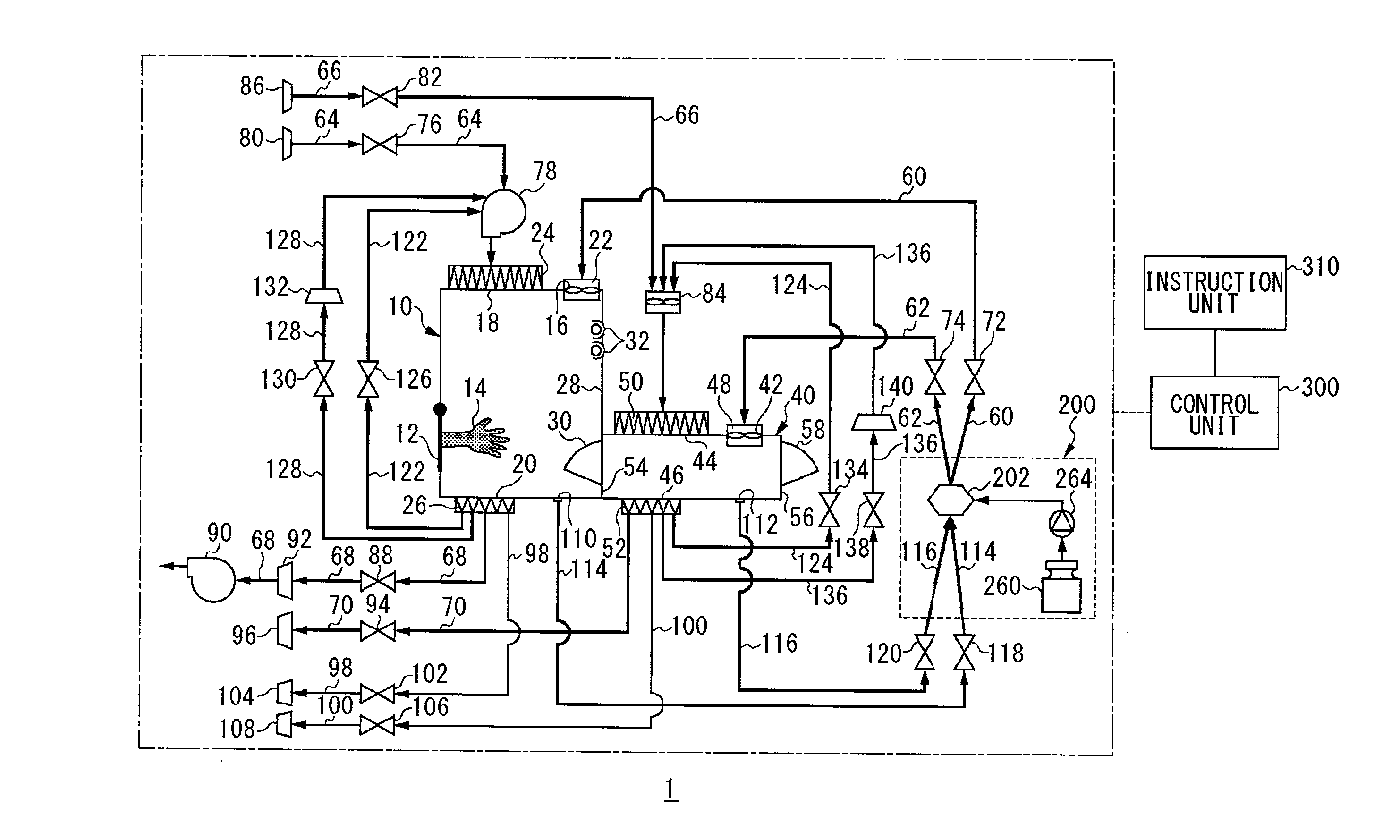
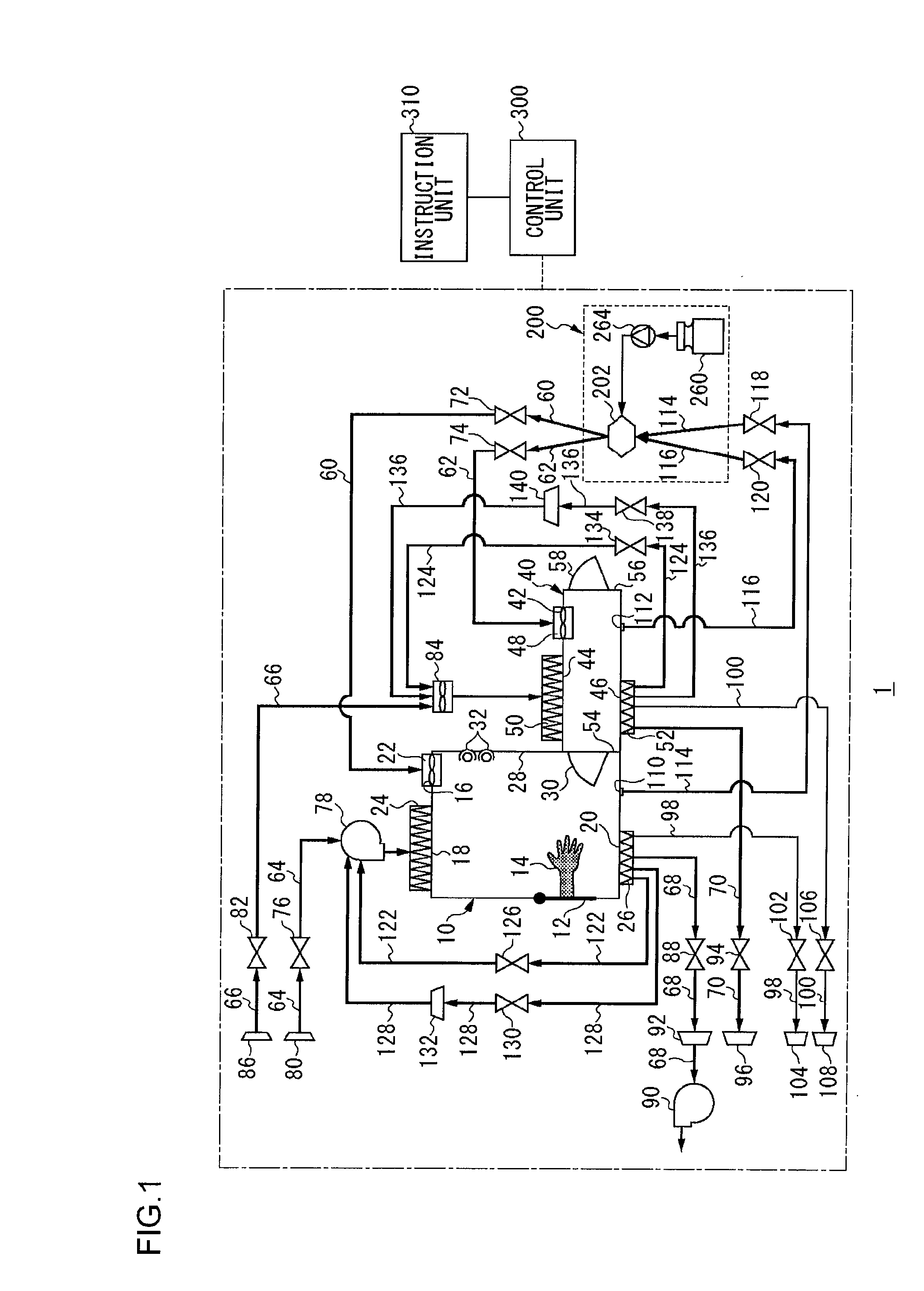
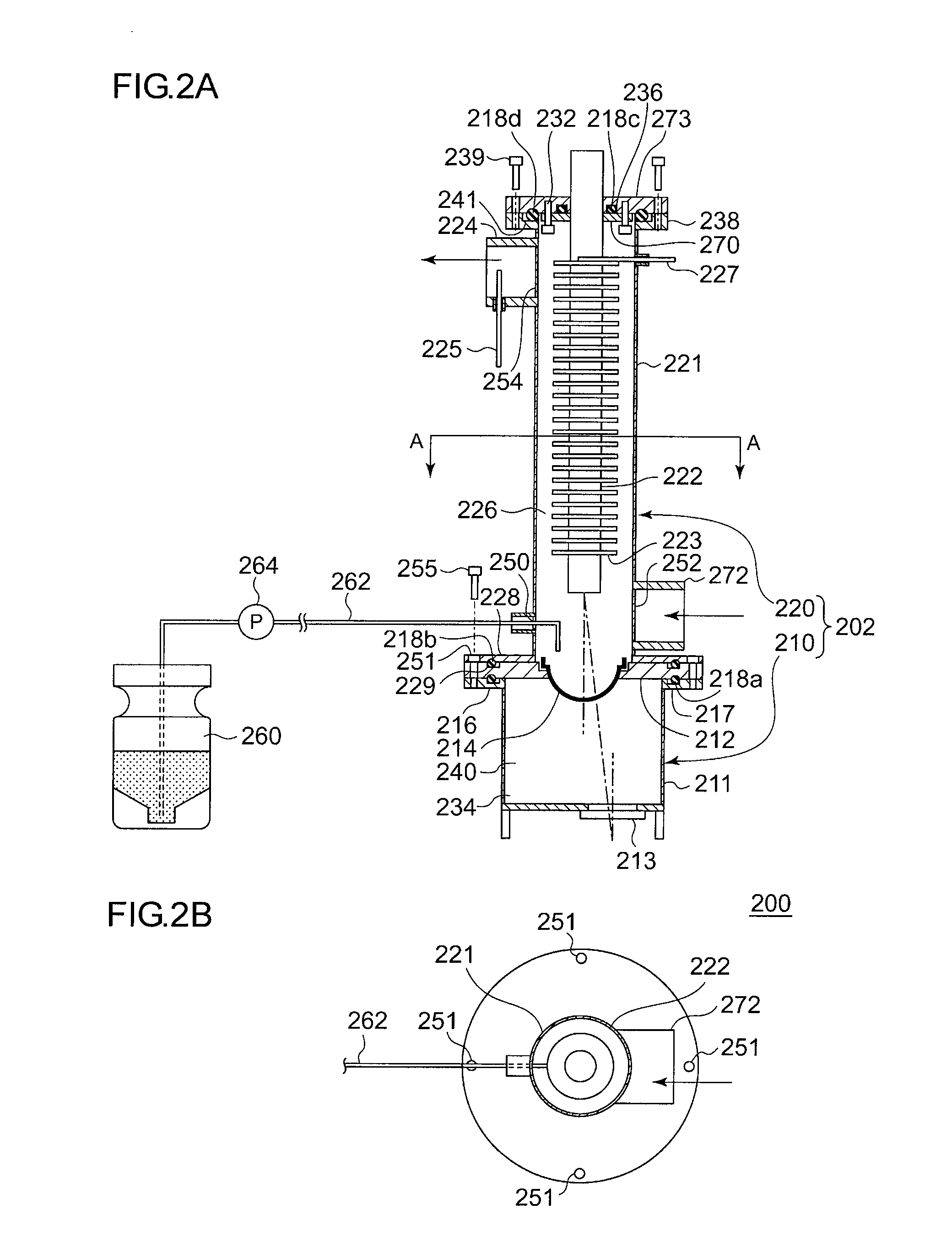
InactiveUS20100189607A1Shorten the timeApparatus sterilizationLighting and heating apparatusProcess engineeringMaterial supply
An isolator includes a workroom having a first sterilizing material supply port, a first sterilizing material discharge port, a first gas supply port and a first gas discharge port, a sterilization chamber having a second sterilizing material supply port, a second sterilizing material discharge port, a second gas supply port and a second gas discharge port, a sterilizing material supply unit, a first sterilizing material supply path connecting the sterilizing material supply unit to the first sterilizing material supply port, a second sterilizing material supply path connecting the sterilizing material supply unit to the second sterilizing material supply port, a first circulating path connecting the first sterilizing material discharge port to the sterilizing material supply unit, a second circulating path connecting the second sterilizing material discharge port to the sterilizing material supply unit, a first gas supply path, a second gas supply path, a first gas discharge path, a second gas discharge path, and a control unit.
Owner:PHC HLDG CORP
Fluid over-flow/make-up air assembly for reprocessor
A system for sterilizing or microbially deactivating instruments and devices. The system includes a circulation system for circulating a microbial deactivation fluid through a chamber for containing the instruments and devices. The chamber forms a portion of the circulation system. The system further includes a fluid over-flow / make-up air assembly. The fluid over-flow / make-up air assembly includes a manifold having an inner cavity that is in fluid communication with the circulation system, an overflow port in the manifold, and an overflow valve assembly disposed in the manifold allowing fluid flow from the cavity to the overflow port when a pressure in the cavity exceeds a pressure in the overflow port by a predetermined amount. A filter assembly is attached to the manifold. The filter assembly has a filter valve assembly in communication with the cavity. The filter assembly is operable to allow air through the filter assembly into the cavity when the pressure within the cavity is a predetermined amount less than the pressure within the filter assembly.
Owner:AMERICAN STERILIZER CO
Regenerative peritoneal dialysis system
PendingUS20170281847A1Organic active ingredientsInorganic active ingredientsEngineeringMechanical engineering
Systems and methods of generating and regenerating peritoneal dialysate are provided. The systems and methods use a dialysate regeneration module, a sterilization module and concentrates to prepare peritoneal dialysate from used peritoneal dialysate or source water. An optional integrated cycler for direct infusion of the generated peritoneal dialysate is included. Optional dialysate storage containers are provided for storage of the peritoneal dialysate prior to use.
Owner:MOZARC MEDICAL US LLC
Sterilization container filter system
InactiveUS7001441B2Reduce and eliminate needAvoid possibilityCombination devicesDiagnosticsSurgical treatmentLocking mechanism
The invention described herein relates to a filter system for sterilization containers which secures a gas permeable filter onto a vented portion of the container. The filter system of the invention includes a locking mechanism that is comfortable and easy to use, and that requires a simple sliding movement of the users hand to lock and unlock the filter system components in place. The invention further includes a filter system structured such that the openings of the filter system plates permit transport of air across the filter while at the same time the openings are arranged to reduce or prevent undesirable physical perforation of the filter. By virtue of its structure, the need for particular rotational positioning of filter components in relation to one another is significantly reduced. The filter system of the invention also reduces or eliminates the need for perimeter gaskets. The invention is useful in a variety of medical applications wherein sterilization of medical devices and instruments is associated with a surgical treatment or procedure.
Owner:CAREFUSION 2200 INC
Bacteriostatic filter cartridge
InactiveUS6854601B2Reduce bacteria countSafely and effectively and economically filterUltrafiltrationReverse osmosisYarnPolyester
A bacteriostatic filter cartridge having a porous core member about which is layered a yarn and / or a polyester membrane and / or melt blown web of polypropylene and / or a trilaminate polypropylene membrane, any or all of which may be impregnated with an antimicrobial agent. The filter cartridge is sized so as to fit tightly into a cartridge housing of a fluid filtration system. Fluid passing through the cartridge housing will be filtered by the filter cartridge to remove contaminants from the water and which prevents the growth of bacterial and other microorganisms on the filter media.
Owner:MICROBAN PROD CO INC
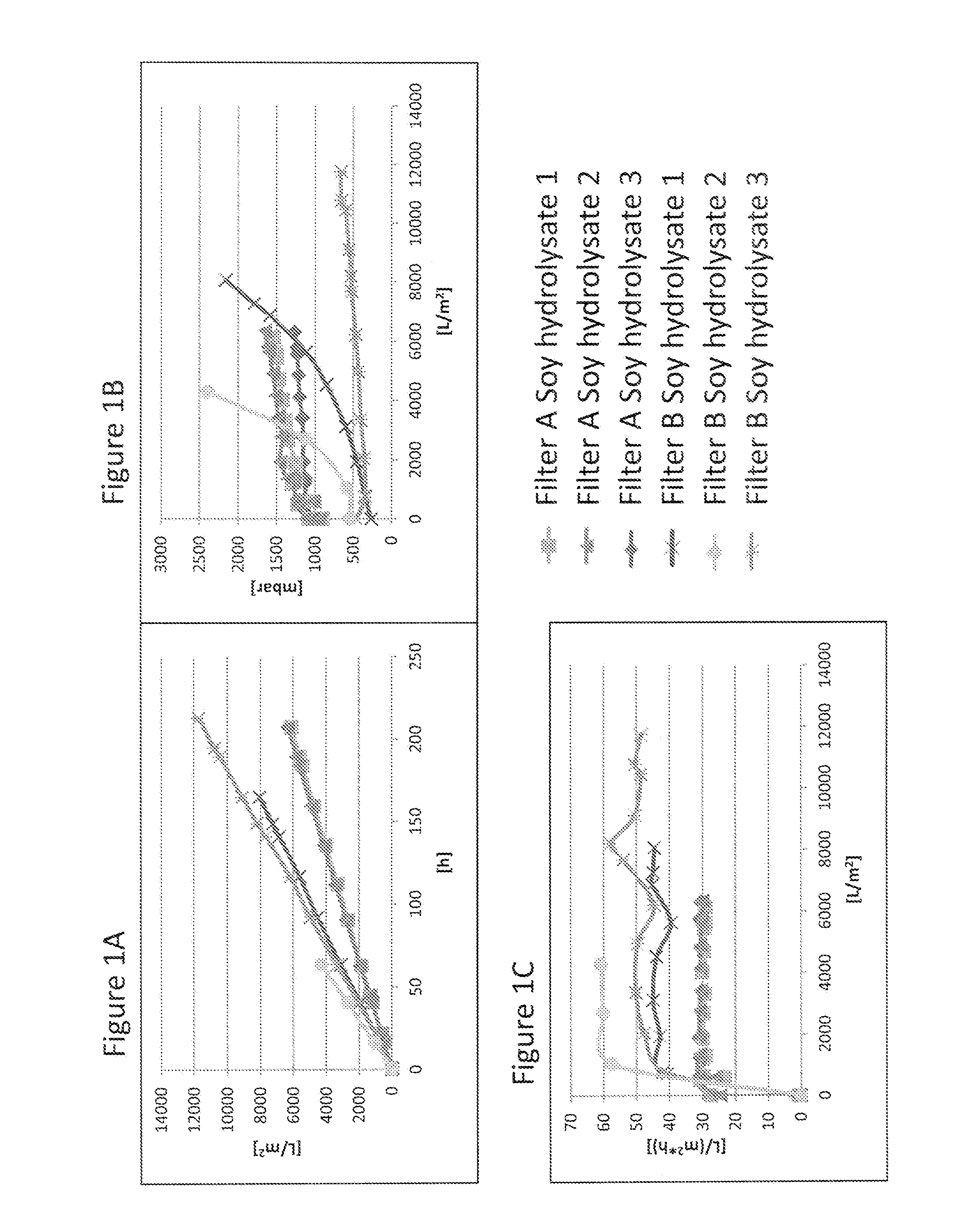
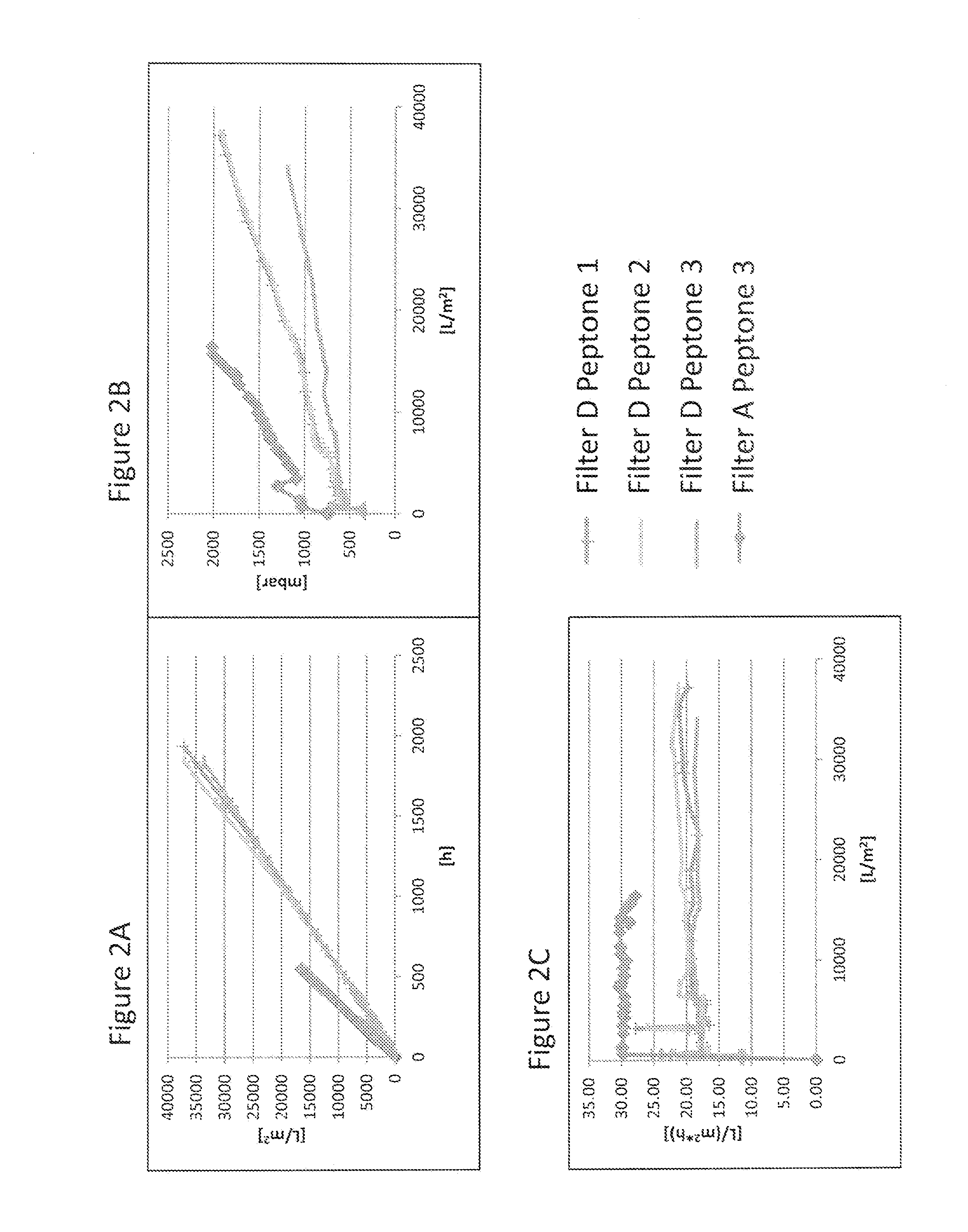
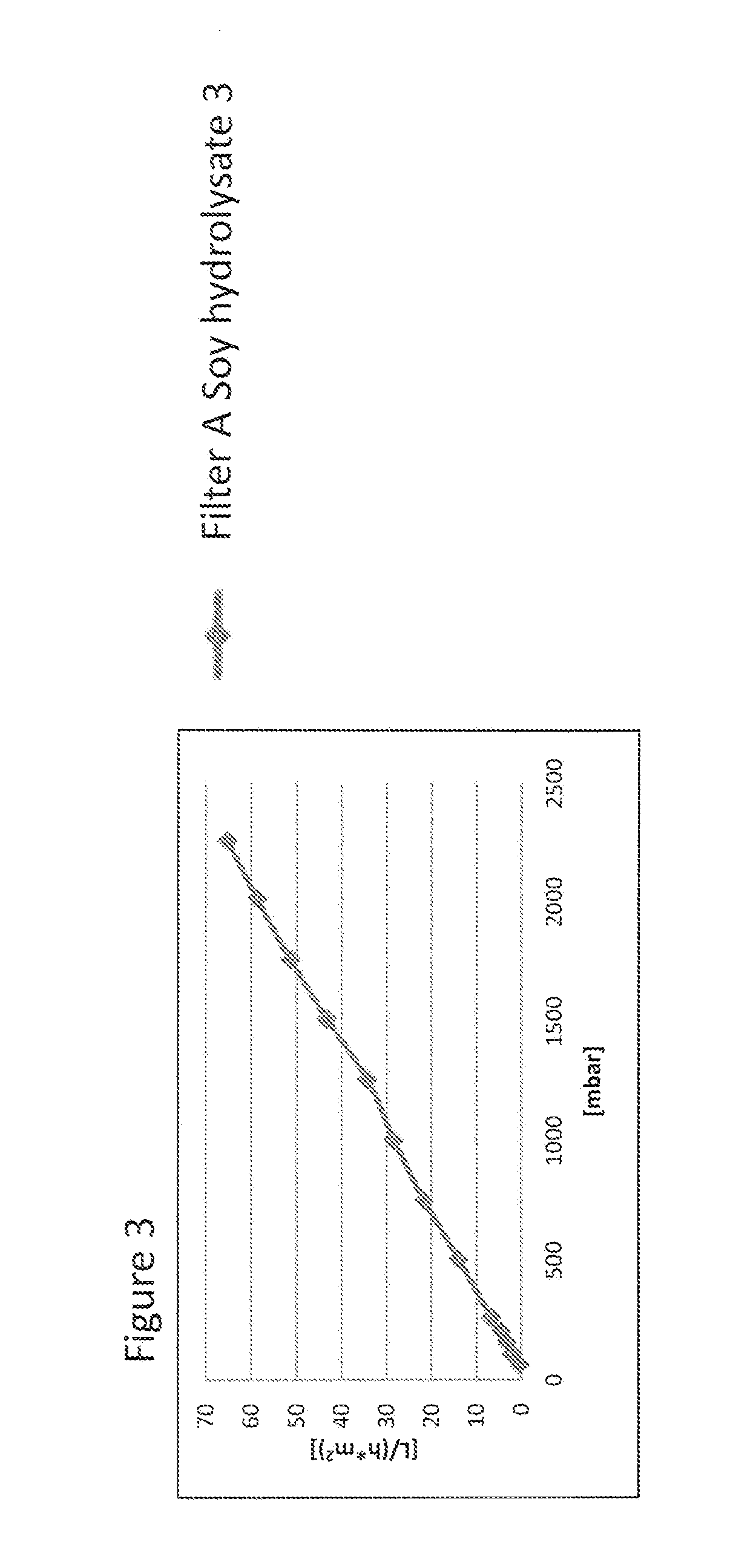
Virus Filtration of Cell Culture Media
ActiveUS20130344535A1Improve virological safetyImprove securityApparatus sterilizationCell culture mediaFiltrationCell culture media
The invention relates to a method for removing a viral contaminant from a preparation, being a cell culture medium or at least a component of a cell culture medium. The method comprises subjecting said preparation to filtration for at least about 24 hours through a virus filter having an effective pore size of maximum about 75 nm. Further, the invention relates to the use of a virus filter in filtration of at least about 24 hours, wherein the virus filter has an effective pore size of maximum about 75 nm for the removal of viral contaminant from a preparation, being a cell culture medium or at least a component of a cell culture medium. In some embodiments the filtration according to the invention operates at a volumetric capacity of at least about 2000 L / m2. Further, the invention relates to the use of a preparation, being a cell culture medium or at least a component of a cell culture medium obtainable according to method of the invention for cell culture; pharmaceutical, diagnostic and / or cosmetic preparations as well as in food preparations.
Owner:TAKEDA PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com