A cyclomalonamide compound with anti-tumor activity and its preparation method and application
A technology of cyclomalonamide and anti-tumor activity, applied in the field of biomedicine, can solve the problems of chemical drugs that cannot achieve therapeutic effects, hair loss, etc., and achieve the effect of inhibiting growth and migration, inhibiting proliferation and migration, and inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
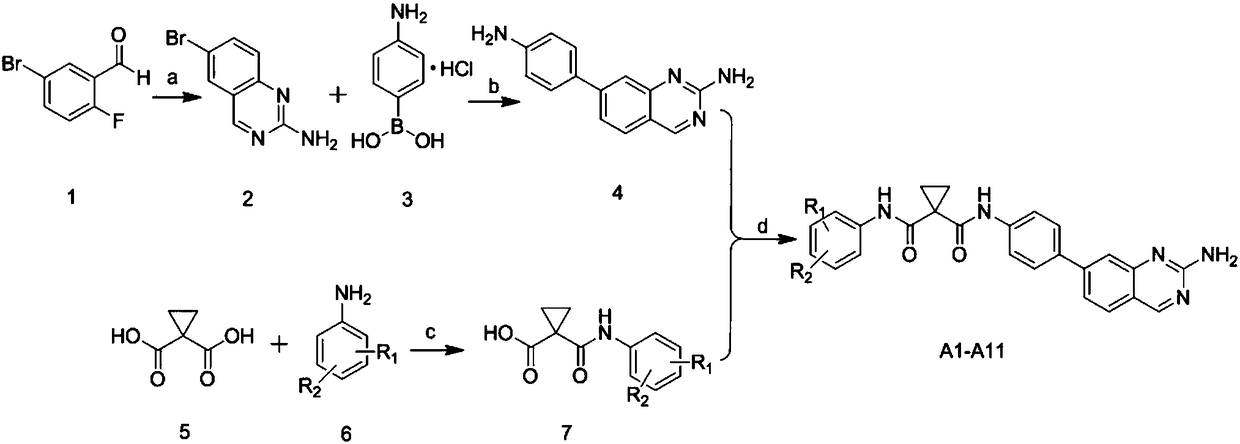
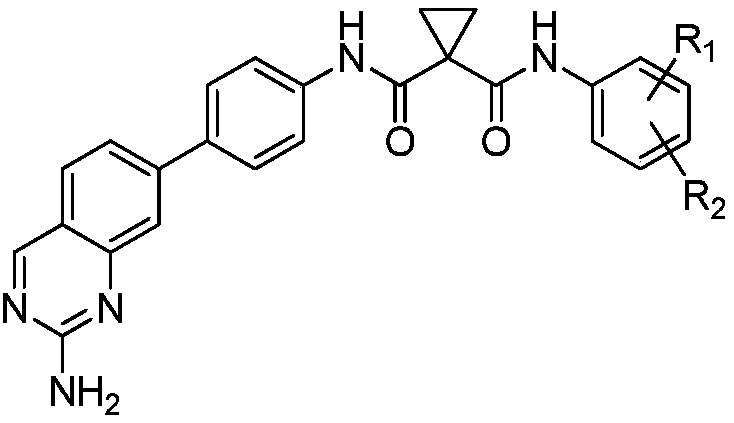
[0034] In the structural formula of the cyclomalonamide compound with anti-tumor activity, R 1 for CF 3 , R 2 is Br, prepared by the following steps (see figure 1 ):
[0035] 1) Preparation of 7-bromoquinazolin-2-amine (compound 2) from 2-fluoro-5-bromobenzaldehyde (compound 1)
[0036] Dissolve 10g (49.2mmol) of 2-fluoro-5-bromobenzaldehyde and 13.3g (74mmol) of guanidine carbonate in N,N-dimethylacetamide solution, reflux at 140°C for 5h, after the reaction, the reaction solution Cool to room temperature, add 120mL of water, a large amount of solids are precipitated, filter with suction, and the obtained filter cake is 7-bromoquinazolin-2-amine, about 5g, yield 60%;
[0037] 2) Preparation of 7-(4-aminophenyl)quinazoline-2 from 7-bromoquinazolin-2-amine (compound 2) and p-aminophenyl borate hydrochloride (compound 3) by Suzuki coupling reaction -amine (compound 4)
[0038] 2g (8.9mmol) 7-bromoquinazolin-2-amine, 1.54g (8.9mmol) p-aminophenyl borate hydrochloride, 2.8g ...
Embodiment 2
[0048] In the structural formula of the cyclomalonamide compound with anti-tumor activity, R 1 , R 2 for chlorine.
[0049] Step 1) to step 2) are the same as steps 1) to 2) of Example 1, that is, preparing 7-bromoquinazolin-2-amine (compound 2) from 2-fluoro-5-bromobenzaldehyde (compound 1) , and then prepared 7-(4-aminophenyl)quinazoline-2 by Suzuki coupling reaction from 7-bromoquinazolin-2-amine (compound 2) and p-aminophenyl borate hydrochloride (compound 3) - Amines (Compound 4).
[0050] 3) Preparation of 1-({3,4-dichloroanilineamino}carbonyl)cyclopropanecarboxylic acid by condensation reaction of 1,1-cyclopropanedicarboxylic acid (compound 5) and 3,4-dichloroaniline (compound 6) (compound 7)
[0051] Under nitrogen protection conditions, 12.9 mL of anhydrous triethylamine was added dropwise to 4 g (30.8 mmol) of 1,1-cyclopropanedicarboxylic acid in dichloromethane solution, stirred for 30 min under ice bath conditions, and then slowly added dropwise 2.3 mL SOCl 2...
Embodiment 3
[0059] In the structural formula of the cyclomalonamide compound with anti-tumor activity, R 1 , R 2 for chlorine.
[0060] Step 1) to step 2) are the same as steps 1) to 2) of Example 1, that is, preparing 7-bromoquinazolin-2-amine (compound 2) from 2-fluoro-5-bromobenzaldehyde (compound 1) , and then prepared 7-(4-aminophenyl)quinazoline-2 by Suzuki coupling reaction from 7-bromoquinazolin-2-amine (compound 2) and p-aminophenyl borate hydrochloride (compound 3) - Amines (Compound 4).
[0061] 3) Preparation of 1-({2,4-dichloroanilineamino}carbonyl)cyclopropanecarboxylic acid by condensation reaction of 1,1-cyclopropanedicarboxylic acid (compound 5) and 2,4-dichloroaniline (compound 6) (compound 7)
[0062] Under nitrogen protection conditions, 12.9 mL of anhydrous triethylamine was added dropwise to 4 g (30.8 mmol) of 1,1-cyclopropanedicarboxylic acid in dichloromethane solution, stirred for 30 min under ice bath conditions, and then slowly added dropwise 2.3 mL SOCl 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com