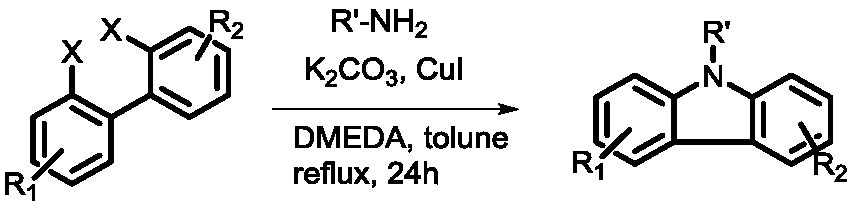
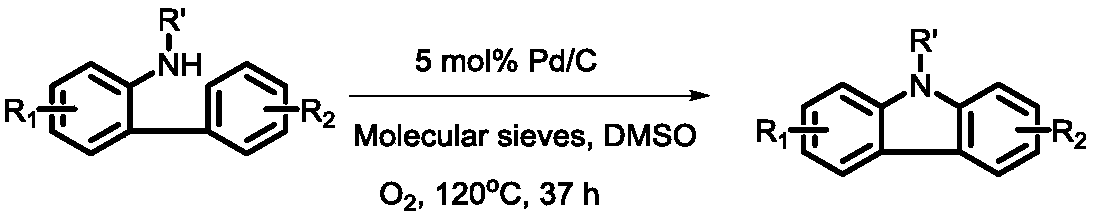
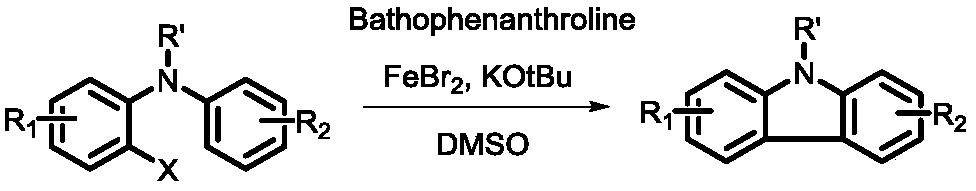
A kind of synthetic method of n-substituted carbazole
A synthesis method and carbazole technology are applied in the field of synthesis of high-value chemical fragments-carbazole compounds, which can solve problems such as unfavorable large-scale production, influence of drugs and optoelectronic materials, expensive phenanthroline and ferrous bromide, etc. problem, to achieve the effect of green mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Place (0.224g, 2mmol) potassium tert-butoxide in a dry flask, dissolve (0.309g, 1mmol) 2-iodo-N-methyl-N-phenylaniline and (25mg, 0.4mmol) ethylene glycol In 5mL of dimethyl sulfoxide. The above solution was added dropwise into a dry flask filled with potassium tert-butoxide, and stirred at 30°C for 6 hours. Quench the reaction with saturated sodium chloride, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to remove ethyl acetate. N-methylcarbazole was separated by thin-layer chromatography with a yield of 83.6%.
Embodiment 2
[0033] (0.336g, 3mmol) potassium tert-butoxide was placed in a dry flask, (0.339g, 1mmol) 2-iodo-N-methyl-N-(4-methoxyphenyl)aniline and (15mg, 0.2 mmol) of 1,3-propanediol was dissolved in 5 mL of toluene. The above solution was added dropwise into a dry flask filled with potassium tert-butoxide, and stirred at 50°C for 12 hours. Quench the reaction with saturated sodium chloride, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to remove ethyl acetate. Thin-layer chromatography gave 3-methoxy-9-methylcarbazole with a yield of 76.5%.
Embodiment 3
[0035] (0.560g, 5mmol) potassium tert-butoxide was placed in a dry flask, (0.305g, 1mmol) 2-bromo-N, 4-dimethyl-N-(4-methoxyphenyl) aniline and (44 mg, 0.6 mmol) n-butanol was dissolved in 5 mL of dioxane. The above solution was added dropwise into a dry flask filled with potassium tert-butoxide, and stirred at 100°C for 8 hours. Quench the reaction with saturated sodium chloride, extract three times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to remove ethyl acetate. Thin-layer chromatography gave 3-methoxy-6,9-dimethylcarbazole with a yield of 63.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com