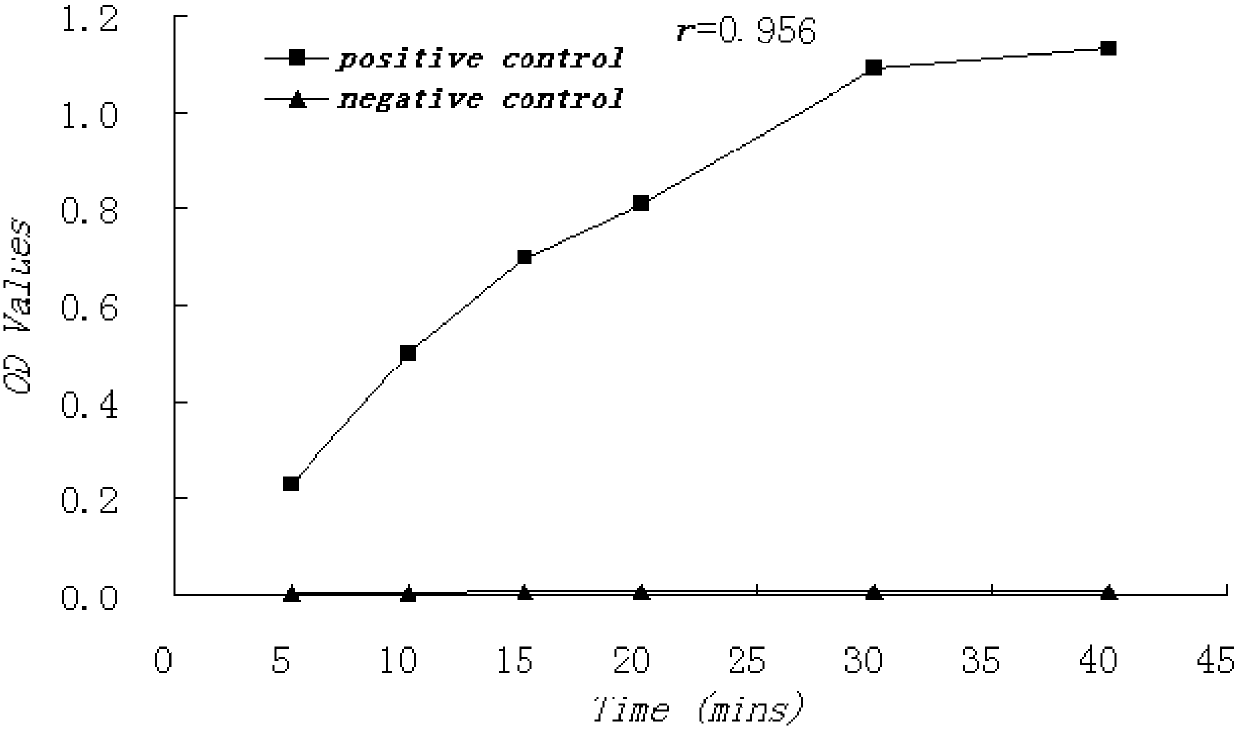
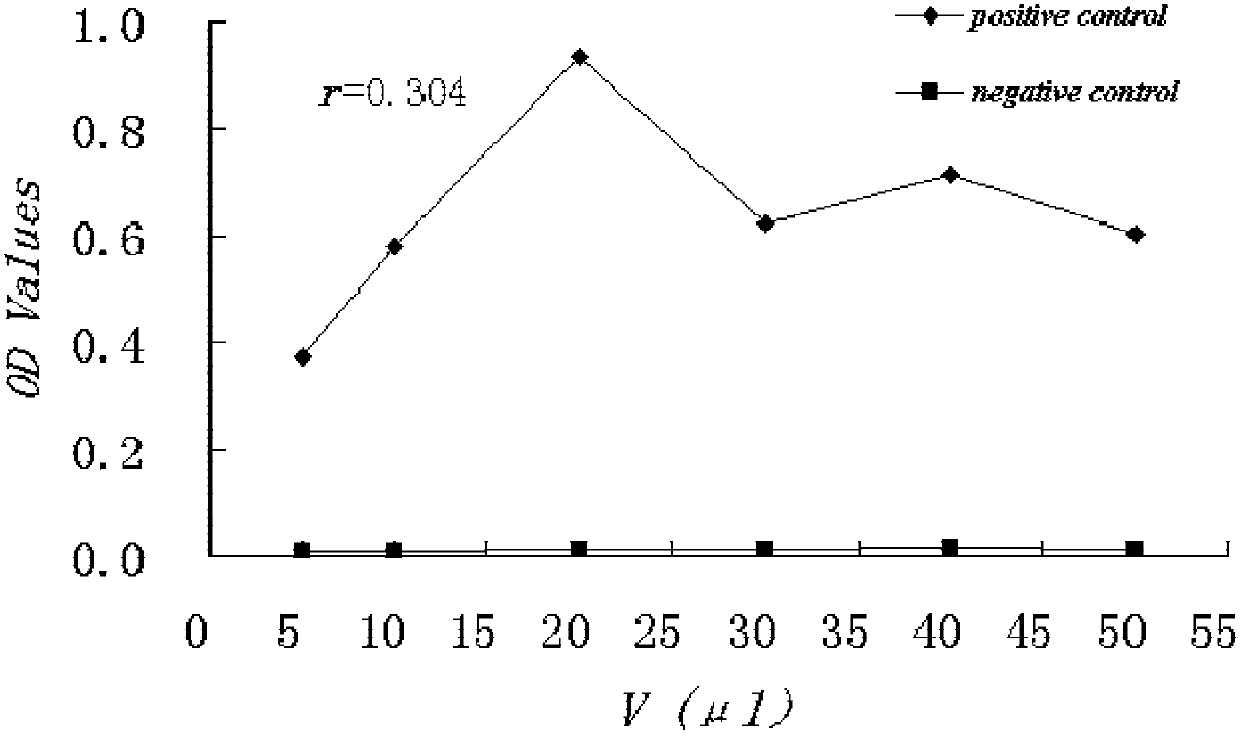
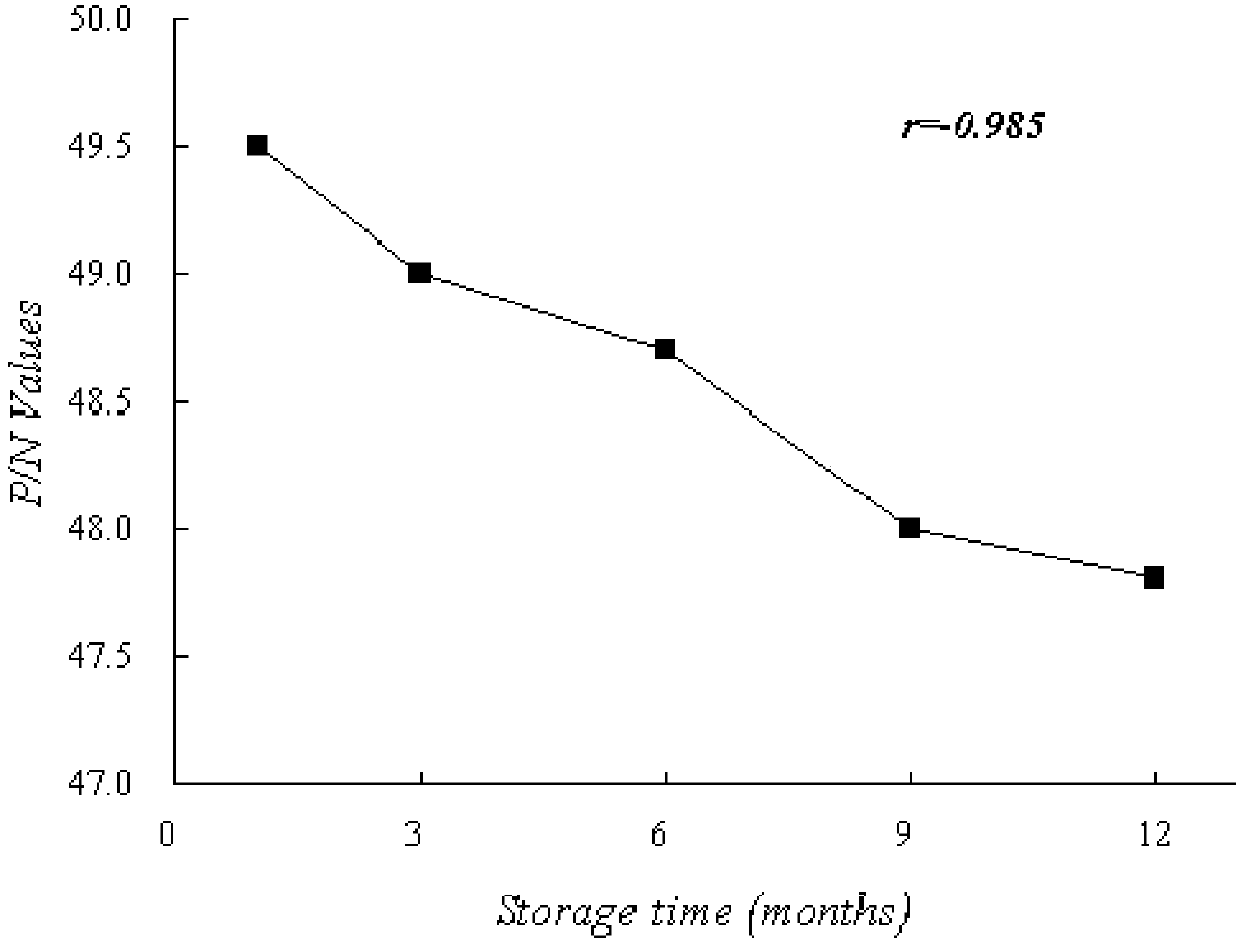
HCV NS3 Monoclonal Antibody and Hepatitis C Detection Kit
A monoclonal antibody, hepatitis C technology, applied in measuring devices, anti-viral immunoglobulins, instruments, etc., can solve the problems of HCAgNS3 detection rate limitation, reduce steric hindrance effect, easy and fast operation, and improve sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The establishment of embodiment 1HCV NS3 monoclonal antibody
[0029] 1. Antigen preparation:
[0030] (1) Construction of the expression plasmid: use the full-length sequence of HCV NS3 as a template, use the following primers, synthesize gene fragments by PCR, perform gel recovery and purification of PCR products, transform and connect them into PMD18T (sequencing vector) by conventional methods, and perform sequencing verification .
[0031] Primer design and cloning: Design upstream and downstream primers according to the full-length sequence of human HCV NS3 in GenBank (FJ890494.1), as shown in SEQ ID No.1 and SEQ ID No.2:
[0032] Upstream SEQ ID No.1: atgctaggcaccataatcacaa
[0033] Downstream SEQ ID No.2: cactgctttatccacacccgg
[0034] The PCR reaction cycle parameters are: 94°C, 4min; 94°C, 30s; 55°C, 30s; 72°C, 1min, 20 cycles, 72°C extension 4min.
[0035] (2) Expression and purification of target protein: HCV NS3 gene (465bp) was amplified with primers c...
Embodiment 2
[0063] Embodiment 2 Preparation of nano magnetic beads hepatitis C detection kit of the present invention
[0064] 1. Preparation of Immunomagnetic Beads (MIB)
[0065] The microspheres used in this example can be patent No. ZL201010168903.2, the microspheres described in the patent name "A Method for Synthesizing Magnetic Polymer Microspheres", or other commercially available superparamagnetic polymer microspheres .
[0066] Take 1 mg streptavidin-modified magnetic microspheres, wash once with MIB washing buffer, add 100 μl biotin-labeled HCV NS3 monoclonal antibody HCV4, mix well, react at room temperature for 30 minutes, magnetically separate, absorb the supernatant, and measure protein concentration, washed three times with MIB washing buffer, added 1ml MIB storage buffer, and stored at 4°C for later use.
[0067] 2. Identification of the coupling efficiency of antibodies on the surface of immunomagnetic beads
[0068] The OD value of the antibody solution (pre) before ...
Embodiment 4
[0082] Embodiment 4 The application experiment verification of the hepatitis C detection kit of the present invention in clinical specimen detection
[0083] 1. Detection of 3708 HCV antibody-negative serum samples:
[0084] HCV antibody was completed by Wantai Hepatitis C-Hepatitis C Virus (HCV) Antibody Diagnostic Kit (Double Antigen Sandwich Enzyme Linked Immunoassay, S20130002), collected from Beijing You'an Hospital from January 2003 to December 2005 The serum specimen (-80 ℃ preservation) that stays, wherein 48 examples detect HCV NS3 antigen-positive (1.3%), and the detection OD value of antigen-positive patient is shown in Table 8 and is that kit of the present invention detects 48 routine HCV antibody negative, HCV NS3 OD value of antigen-positive patients (>0.168 is positive).
[0085] Table 8 HCV Antibody Negative Serum Sample HCV NS3 Antigen Positive Detection Results
[0086]
[0087]
[0088] 2. HCV NS3 antigen detection in 173 HCV antibody-positive patient...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com