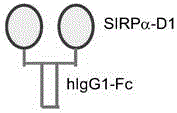Novel recombinant bifunctional fusion protein as well as preparation and application thereof
A fusion protein and dual-function technology, which can be applied in the direction of drug combination, fusion polypeptide, peptide/protein components, etc., can solve the problem of insufficient CD47 affinity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Experimental materials and methods
[0075] 1. Construction of SIRPα-Fc and SIRPαD1-Fc expression vectors
[0076] The expression vector used was pIg-Tail (R&D Systems). Use primer 1 (SEQ ID No.: 9) and primer 2 (SEQ ID No.: 10), primer 1 (SEQ ID No.: 9) and primer 3 (SEQ ID No.: 11) respectively the outer membrane of SIRPα The end and SIRPαD1 gene coding sequence from THP-1 ( TIB-202 TM ) cells, and the PCR products were respectively cloned into the HindIII / EcoRI sites of the improved pIg-Tail vector to form pSIRPα-Fc and pSIRPαD1-Fc vectors.
[0077] 2. Construction of HY03M and HY03MM expression vectors
[0078] The SIRPαD1 gene sequence carrying the N89A mutant was sent to Nanjing GenScript Synthesis (Project No.: 7009323-1), and the synthesized gene was cloned into the HindIII / EcoRI site of the pIg-Tail vector to form the HY03M expression vector. Using primer 4 (SEQ ID No.: 12) and primer 5 (SEQ ID No.: 13), through site-directed mutagenesis PCR, the gene seq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com