Staphylococcus aureus sortase a high efficiency mutant
A staphylococcus and mutant technology, applied in the field of bioengineering, can solve problems such as low catalytic efficiency, and achieve the effect of improving catalytic efficiency and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Construction of Staphylococcus aureus sortase A random mutation library using error-prone PCR
[0035] Using error-prone PCR to introduce nucleotide mutations into the Staphylococcus aureus sortase A gene in vitro, the specific conditions for error-prone PCR are as follows:
[0036]
[0037]
[0038]The PCR template is a plasmid containing the wild-type Staphylococcus aureus sortase A gene. Firstly, the nucleotide sequence corresponding to the amino acid sequence from the 26th to the 206th position is cloned into between the NdeI and XhoI restriction sites of the pet28a vector by subcloning.
[0039] Upstream primer: 5'-GGAATTCCATATGAAACCACATATCGATAATTATC-3' (SEQ ID NO.11)
[0040] Downstream primer: 5'-GGTAGGCACTCGAGTTATTTGACTTCTGTAGCTAC-3' (SEQ ID NO.12)
[0041] PCR amplification conditions: 95°C for 5 min; 95°C for 1 min, 55°C for 1 min, 72°C for 1 min, 30 cycles; 72°C for 10 min.
[0042] The error-prone PCR amplification product was purified by...
Embodiment 2
[0043] Example 2, Construction of Staphylococcus aureus sortase A site saturation mutation library by point mutation PCR
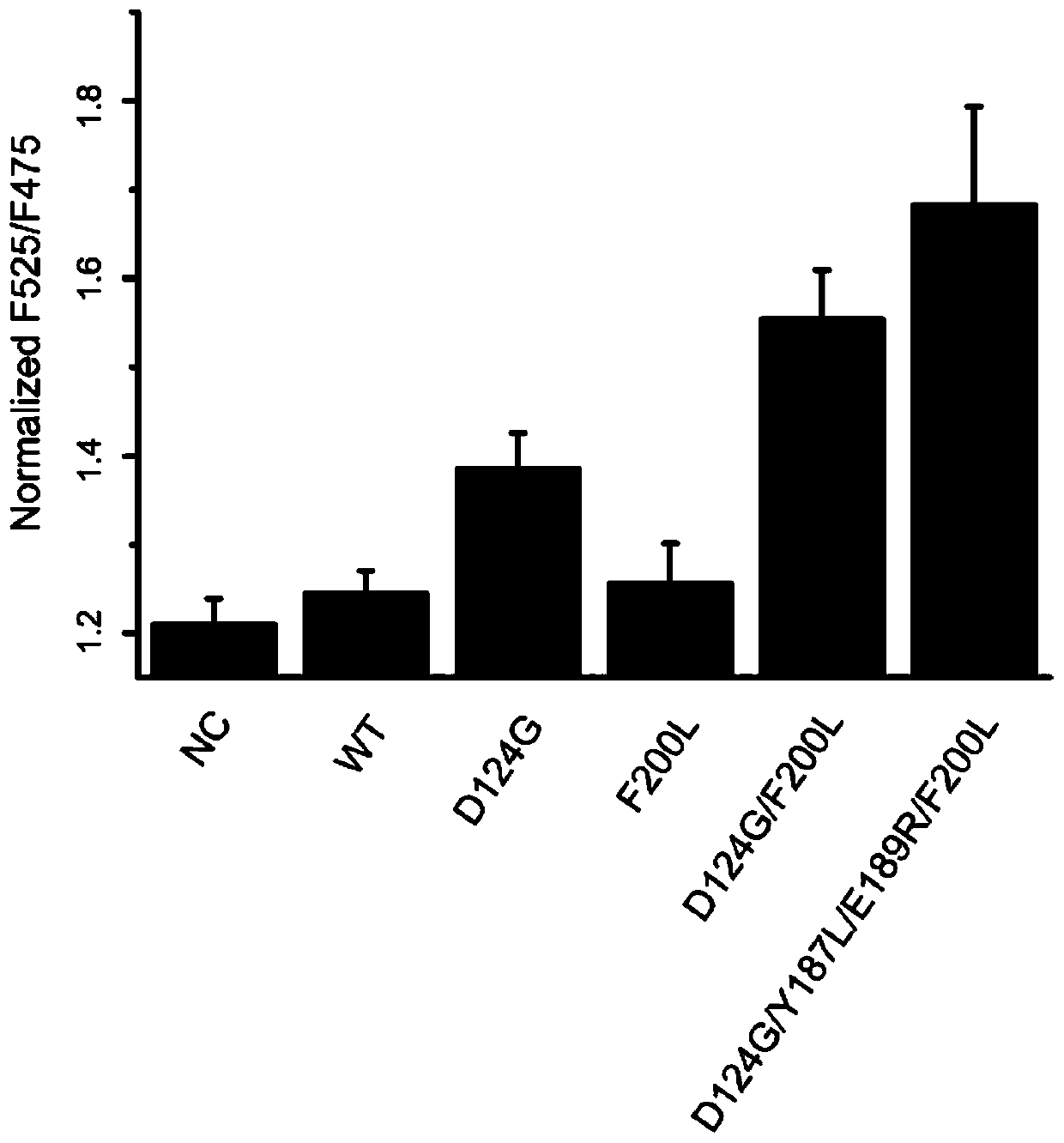
[0044] Use the point mutation PCR method to introduce saturation mutations into the two sites Tyr187 and Glu189 of the P94R / D160N / D165A / K190E / K196T mutant of sortase A, and the PCR conditions are as follows:
[0045]
[0046]
[0047] The PCR template is the gene of sortase 2, which is obtained by mutating the pet28a plasmid containing the wild-type sortase A gene in Example 1.
[0048] Upstream primer: 5'-CATTAATTACTTGTGATGATNNKAATNNKGAGACAGGCGTTTGGG-3' (SEQ ID NO.13)
[0049] Downstream primer: 5'-CCCAAACGCCTGTCTCMNNATTMNNATCATCACAAGTAATTAATG-3' (SEQ ID NO.14)
[0050] In the above primers, N represents one of the four bases A, C, G, and T, K represents G or T, and M represents A or C.
[0051] PCR amplification conditions: 95°C for 5min; 95°C for 45s, 55°C for 45s, 72°C for 7min, 23 cycles; 72°C for 10min.
[0052] The point mutation PCR produc...
Embodiment 3
[0053] Example 3. Construction and verification of a screening system based on fluorescence resonance energy transfer
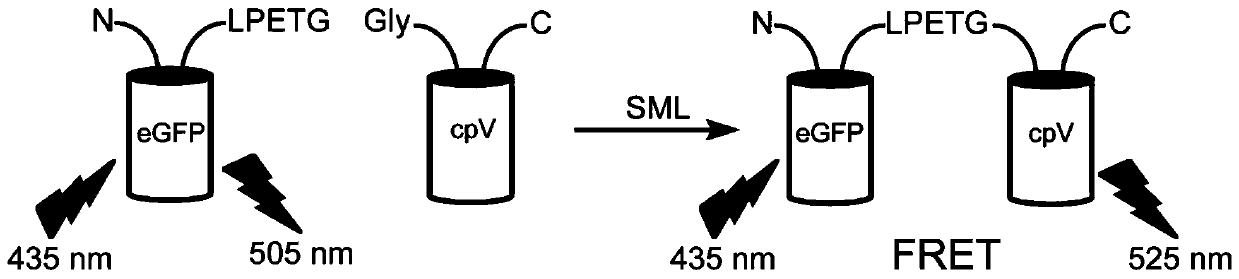
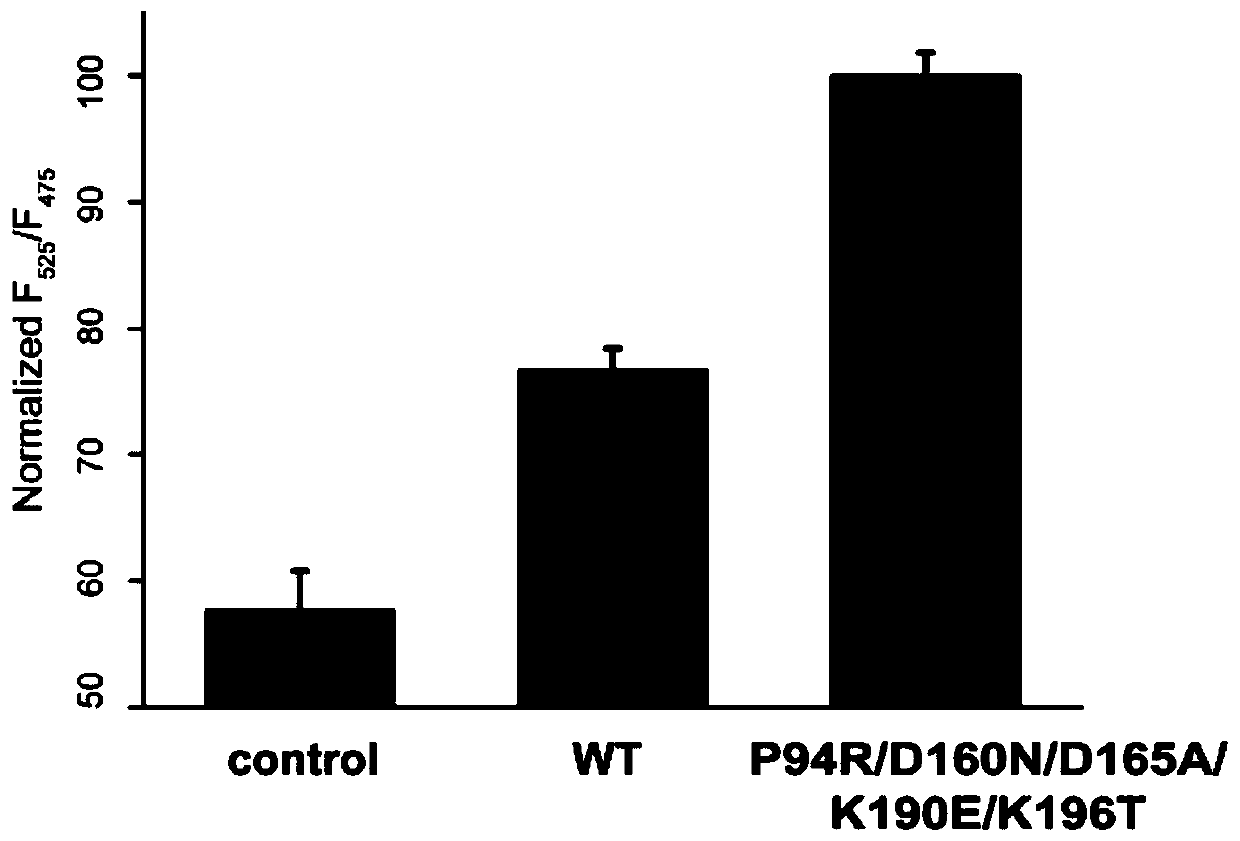
[0054] Using the method of subcloning, the fluorescent proteins of eGFP-LPETG and G-cpV were fused and expressed on the pet28a vector, purified using 6×His tags, and the purified proteins were subjected to a sortase-mediated ligation reaction, such as figure 1 As shown, EGFP and cpV proteins are suitable donors and acceptors of fluorescence resonance energy transfer (FRET). The fluorescence resonance energy transfer efficiency between proteins is improved, and the higher the sortase activity is, the higher the fluorescence function energy transfer efficiency is. Validation of the screening platform using wild-type sortase A and departure sortase 2 demonstrated that sortase A activity is directly proportional to fluorescence resonance energy transfer efficiency, as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com