A small molecule inhibitor of Ebola pseudovirus
An Ebola virus and inhibitor technology, applied in the field of small molecule inhibitors of Ebola pseudovirus, can solve problems such as unapproved vaccines and drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
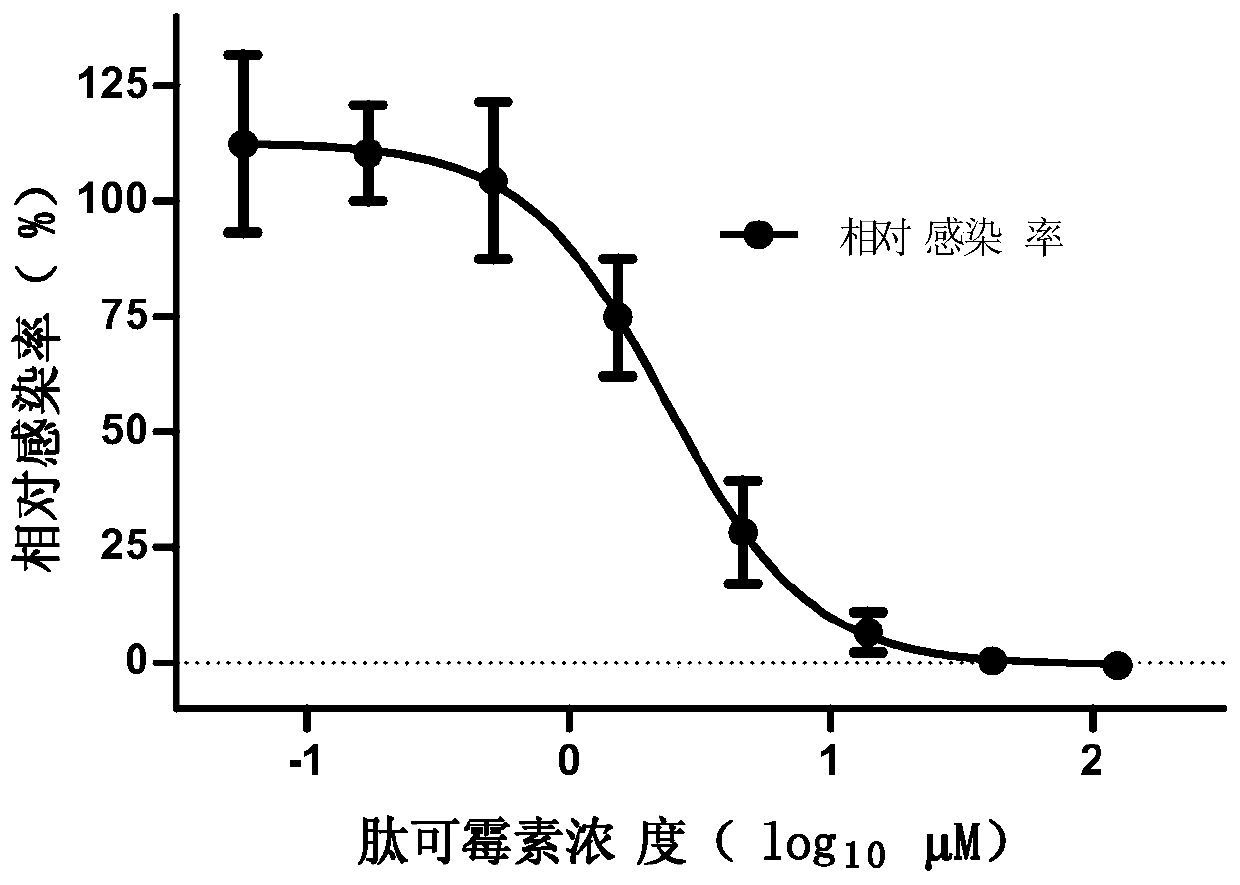
[0092] Peptokomycin can inhibit the infection of cells by Zaire Ebola pseudovirus, and its EC 50 was 2.38 μM. And it does not show cytotoxicity at the highest tested concentration (125 μM). Peptokomycin was not able to inhibit the infection of cells by vesicular stomatitis pseudovirus.
[0093] Figure 1. Peptokomycin inhibits the infection of cells by Zaire-type Ebola pseudovirus. Figure 1A Add 3-fold diluted peptokomycin to Vero cells, add Zaire-type Ebola pseudovirus and culture for 72 hours, then use the Bright-Glo kit to detect luciferase, and test the effect of peptokomycin on Zaire Inhibitory effect of Ebola pseudovirus infection. Figure 1B Add 3-fold diluted peptokomycin to the Vero cells, add the culture medium for 72 hours, and then use the CellTiter-Glo kit to detect the cell viability and test the toxicity of peptokomycin to the cells. Figure 1C Add 3-fold diluted pepticomycin to Vero cells, add vesicular stomatitis pseudovirus and culture for 72 hours, then...
Embodiment 2
[0098] Peptokomycin had no inhibitory effect on enterovirus 71 and was not cytotoxic to RD cells at a concentration of 125 μM.
[0099] Figure 3 Pepticocomycin does not inhibit enterovirus type 71. Figure 3A Vero cells were infected with EV71 with a multiple of infection (MOI) of 0.1, and pepticomycin at concentrations of 10 μM, 30 μM, and 100 μM were added, and the supernatant was collected after 42 hours of incubation, and the virus titers were determined by plaque formation assay. Spend. The data in the figure are from two independent parallel experiments, and the error bars represent the standard deviation of two parallel experiments. Figure 3B RD cells were added 3-fold diluted peptokomycin, added to the culture medium and cultured for 96 h, and the cell viability was detected by the CellTiter-Glo kit to detect the effect of peptokomycin on the cells. The data in the figure are from two independent parallel experiments, and the error bars represent the standard devia...
Embodiment 3
[0101] Peptokomycin prevents adsorption of Zaire-type Ebola pseudoviruses to cells.
[0102] Figure 4 Peptokomycin prevents adsorption of Zaire-type Ebola pseudoviruses to cells. Add pepticomycin with a concentration of 10 μM, 100 μM, and DMEM containing 2% FBS and 1% P / S to the pseudovirus and cells according to three different treatments before adsorption, during infection and after adsorption, and culture for 72 hours Afterwards, the Bright-Glo kit was used to detect the luciferase, and the effect of peptokomycin on different stages of virus entry into cells was evaluated. The data in the figure are from two independent parallel experiments, and the error bars represent the standard deviation of two parallel experiments. Results were processed using Graphpad Prism5. Experimental results showed that pepticomycin could prevent Zaire-type Ebola pseudovirus from adsorbing to cells, but it could not work after pseudovirus was adsorbed to cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com