Application of appa in preparation of medicine for treating diabetic nephropathy
A technology for diabetic nephropathy and drugs, which is applied in drug combinations, urinary system diseases, metabolic diseases, etc., can solve problems such as no related reports, and achieve good effects, good medicinal prospects, and small doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
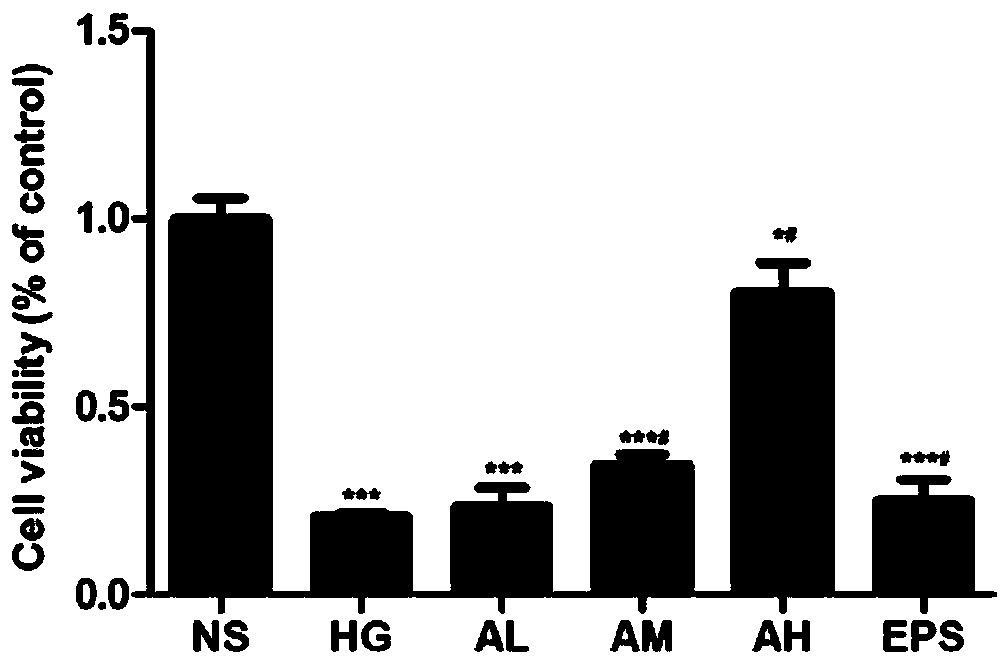
[0034] Example 1 Effect of APPA on proliferation of glomerular mesangial cells (HBZY-1)
[0035] In order to determine the effect of different concentrations of APPA on the proliferation activity of HBZY-1 cells, 6 groups were established (NS: normal control group; HG: high glucose administration model group; AL: APPA low dose (0.3 μ M) administration group; AM : APPA middle dose (0.6 μ M) administration group; AH: APPA high dose (0.9 μ M) administration group; EPS: epalrestat (10 μ M) positive control group), each group is 33mmol / L in sugar concentration and cultivates 72h , each APPA group and the EPS group were administered at the same time, and the absorbance (OD) value at 490 nm of cells in each well was detected by using the MTT method. ;
[0036] Experimental results: attached figure 1 Show: Compared with the NS group, the OD value of each experimental group decreased, HG, AL, AM, EPS group P# P## P<0.01.
Embodiment 2
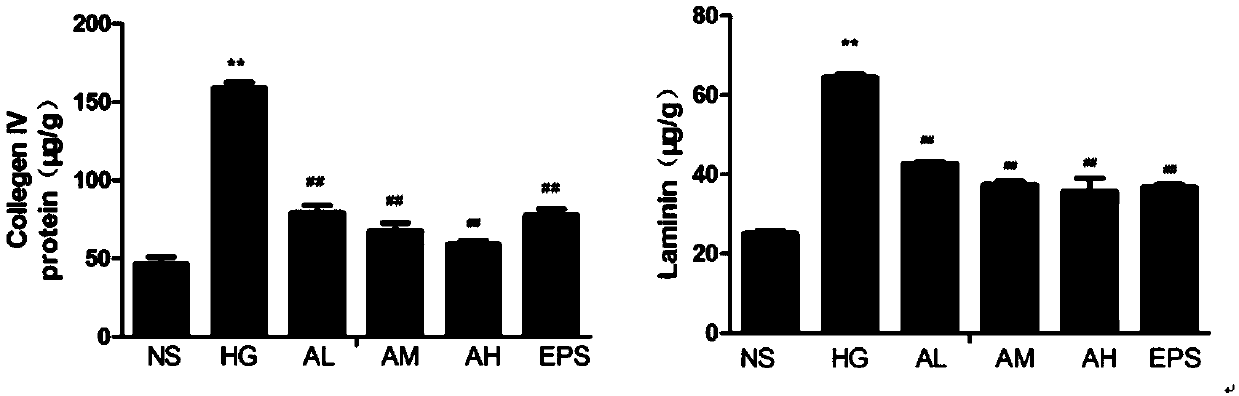
[0037] Example 2 Determination of Cell Type IV Collagen and Laminin Content
[0038] By setting 6 groups (NS: normal control group; HG: high glucose administration model group; AL: APPA low dose (0.3 μM) administration group; AM: APPA medium dose (0.6 μM) administration group; AH: APPA High dose (0.9 μ M) administration group; EPS: epalrestat (10 μ M) positive control group) measure intercellular IV collagen and laminin content, the result is as attached figure 2 Shown: Compared with the normal control group, *P# P## P<0.01; By comparing the normal control group and the high-glucose-induced group, it can be found that high-glucose induction can significantly increase the generation of intercellular matrix hyperplasia, and the content of type IV collagen and laminin in the cell extract is significantly higher than that of the vehicle control group and after APPA administration, it was found that compared with the HG group, the higher the concentration of APPA administration, t...
Embodiment 3
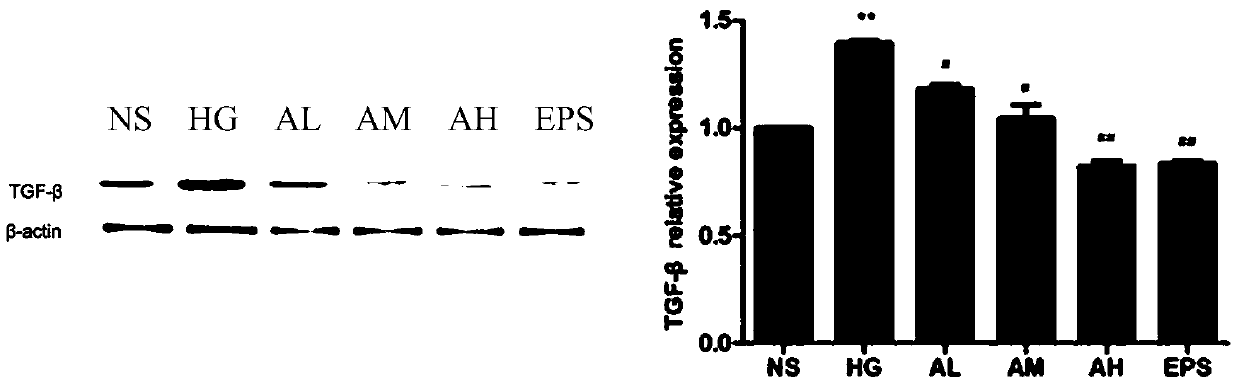
[0039] Example 3 Determination of Cellular TGF-β1 Protein Expression
[0040] This experiment adopts the method of Western Bloot to measure 6 groups (NS: normal control group; HG: high glucose administration model group; AL: APPA low dosage (0.3 μ M) administration group; AM: APPA dosage (0.6 μ M) administration group; AH: APPA high-dose (0.9μM) administration group; EPS: epalrestat (10μM) positive control group) intracellular TGF-β1 protein, as attached image 3 The results showed: compared with the normal control group, *P# P## P<0.01; compared with the NS group, the TGF-β1 protein content in the HG and AL groups significantly increased P<0.05, and compared with the HG group, the TGF-β1 protein content in the AL and AM groups decreased significantly P <0.05, the decrease of TGF-β1 content in EPS group was the most significant P<0.01.
[0041] In conclusion, APPA has a certain inhibitory effect on the HBZY-1 cell injury model induced by high glucose, and can reduce the expre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com