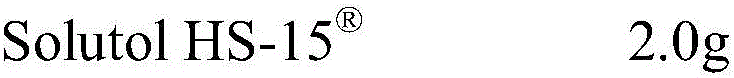Drug composition for improving safety of compound ginseng injection
A composition and injection technology, applied in the field of medicine, can solve the problems of pH value drop of solution, easy rancidity of polysorbate 80, accelerated pH value of liquid medicine, etc., achieve high dosage, improve safety and good effect of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016]
[0017] Preparation method: ginseng and Dan are decocted twice in water for 2 hours each time, combined with decoction, filtered, the filtrate is concentrated under reduced pressure to a relative density of 1.27-1.30 (80°C), let cool, and ethanol is added to make the ethanol content It was 65%, left standing overnight, filtered, the filtrate was decompressed to recover ethanol and concentrated to a relative density of 1.30 (80 ° C), 3 times the amount of water was added, fully stirred, refrigerated for 48 hours, filtered, and the filtrate was concentrated under reduced pressure to a relative density of 65%. The density is about 1.15 (80°C), and the medicinal liquid is used for later use; add water to decoction for two times, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25 to 1.30 (80°C), and let it cool. , add ethanol to make the ethanol content be 65%, let stand overnight, filter, concentrate th...
Embodiment 2
[0019]
[0020] Preparation method: ginseng and Dan are decocted twice in water for 2 hours each time, combined with decoction, filtered, the filtrate is concentrated under reduced pressure to a relative density of 1.27-1.30 (80°C), let cool, and ethanol is added to make the ethanol content It was 65%, left standing overnight, filtered, the filtrate was decompressed to recover ethanol and concentrated to a relative density of 1.30 (80 ° C), 3 times the amount of water was added, fully stirred, refrigerated for 48 hours, filtered, and the filtrate was concentrated under reduced pressure to a relative density of 65%. The density is about 1.15 (80°C), and the medicinal liquid is used for later use; add water to decoction for two times, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25 to 1.30 (80°C), and let it cool. , add ethanol to make the ethanol content be 65%, let stand overnight, filter, concentrate the filtrate u...
Embodiment 3
[0022]
[0023] Preparation method: ginseng and Dan are decocted twice in water for 2 hours each time, combined with decoction, filtered, the filtrate is concentrated under reduced pressure to a relative density of 1.27-1.30 (80°C), let cool, and ethanol is added to make the ethanol content It was 65%, left standing overnight, filtered, the filtrate was decompressed to recover ethanol and concentrated to a relative density of 1.30 (80 ° C), 3 times the amount of water was added, fully stirred, refrigerated for 48 hours, filtered, and the filtrate was concentrated under reduced pressure to a relative density of 65%. The density is about 1.15 (80°C), and the medicinal liquid is used for later use; add water to decoction for two times, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25 to 1.30 (80°C), and let it cool. , add ethanol to make the ethanol content be 65%, let stand overnight, filter, concentrate the filtrate u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com