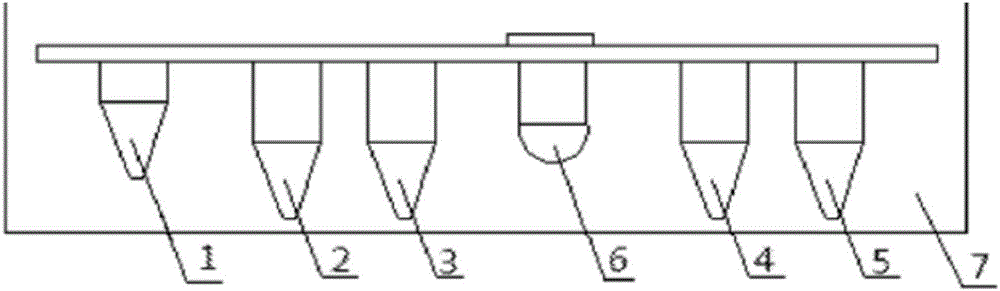
DCP quick separation detection kit
A detection kit and rapid technology, applied in the field of medical devices and in vitro diagnostics, can solve the problems of limited monoclonal antibody recognition epitopes, low detection sensitivity, and inability to capture DCP
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This embodiment provides a DCP rapid separation and detection kit, and provides a preparation method for producing a preferred anti-DCP monoclonal antibody for preparing the kit.
[0056] 1. Preparation of anti-DCP monoclonal antibody:
[0057] The preparation of anti-DCP monoclonal antibodies in this example can be the first antibody, the second antibody or the combination of the first antibody and the second antibody produced by hybridoma cells 4D09 and 3H08, or other commercial anti-DCP monoclonal antibodies Antibody.
[0058] In order to better explain the present invention, the preparation of the anti-DCP monoclonal antibody is preferably the preparation of the first antibody and the second antibody composition; this preparation method is only for better explanation of the present invention, not for the preparation and use of the anti-DCP monoclonal antibody. limitation factor.
[0059] Take hybridoma cells 4D09 and 3H08 (preserved in the General Microbiology Cen...
Embodiment 2
[0086] The invention provides a DCP rapid separation detection kit and its application, which is mainly used for accurate and quantitative detection of DCP content in human serum samples, and the detection results of serum samples are used for primary hepatocellular carcinoma (HCC) and chronic liver disease Differential diagnosis of benign hepatic space-occupying lesions.
[0087] The following detection steps are mainly used to better explain the use and application of the present invention, and are not used as limiting conditions for the application of the present invention.
[0088] The main detection steps are as follows:
[0089] 1. The compatible instrument for Example 2 is: Maglumi series automatic immune analyzer (MQ60 type) produced by Beijing Rejing Biotechnology Co., Ltd.
[0090] 2. MQ60 detection steps:
[0091] (1) Reagent preparation
[0092] a) The kit should be equilibrated to room temperature when it is taken out from the refrigerated environment before us...
Embodiment 3
[0110] A DCP rapid separation detection kit and application thereof of the present invention are mainly used for accurate and quantitative detection of DCP content in human serum samples, and the detection results of serum samples are used for primary hepatocellular carcinoma (HCC) and chronic liver disease and benign Differential diagnosis of hepatic space-occupying lesions.
[0111] The following detection steps are mainly used to better explain the use and application of the present invention, and are not used as limiting conditions for the application of the present invention.
[0112] The main performance indicators are as follows:
[0113] 1. The compatible instrument for Example 2 is: Maglumi series automatic immune analyzer (MQ60 type) produced by Beijing Rejing Biotechnology Co., Ltd.
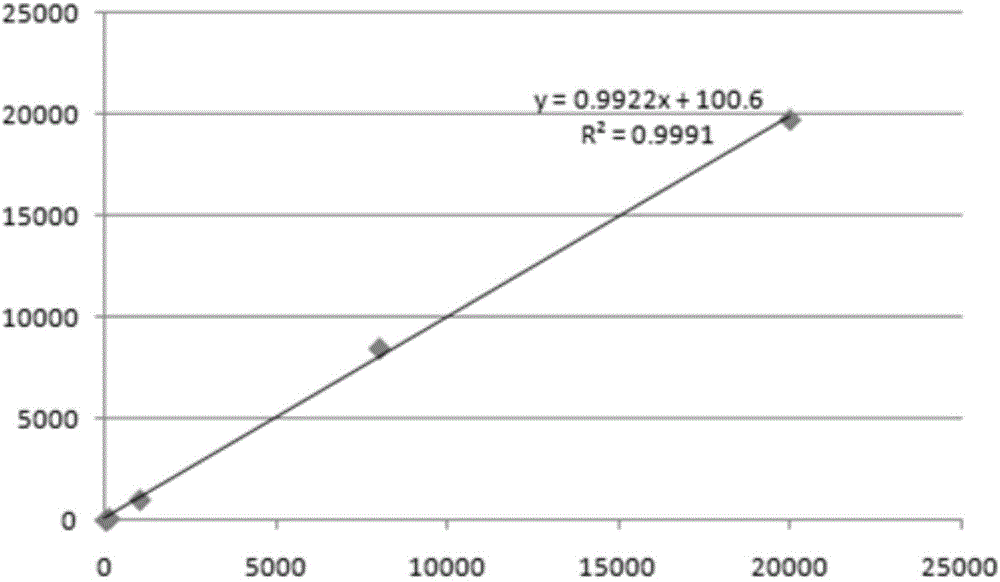
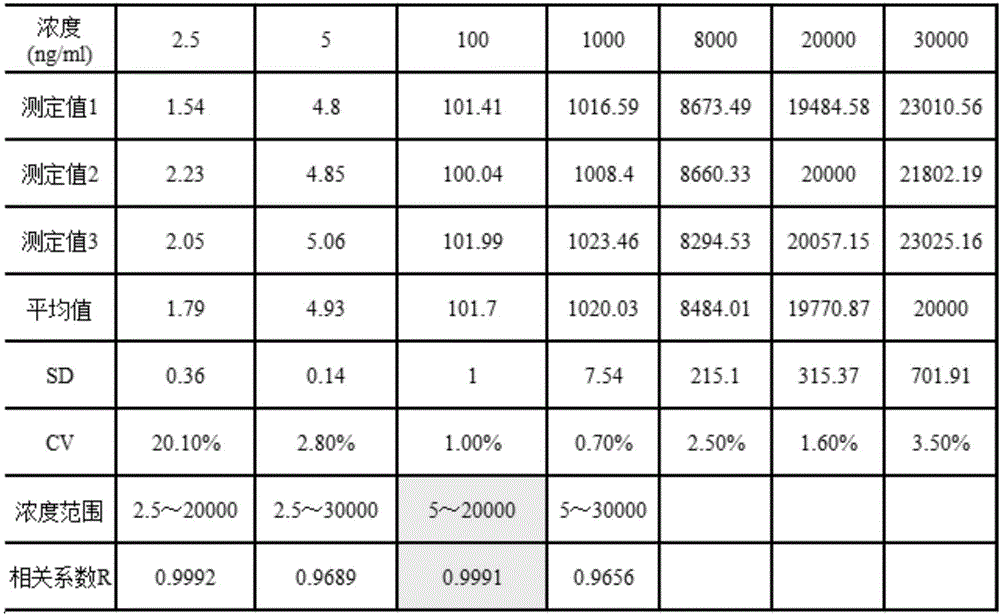
[0114] 2. Minimum detection limit:
[0115] The lowest detection limit of DCP means that the DCP content can be distinguished from the point where the DCP content is zero. The high-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com