Combination of lenalidomide or pomalidomide and CD38 antibody-attenuated interferon-alpha constructs, and the use thereof
A technology of lenalidomide and interferon α, applied in the direction of interferon, cytokines/lymphokines/interferons, antibodies, etc., can solve the problem of undesired activity of IFN on healthy cells and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
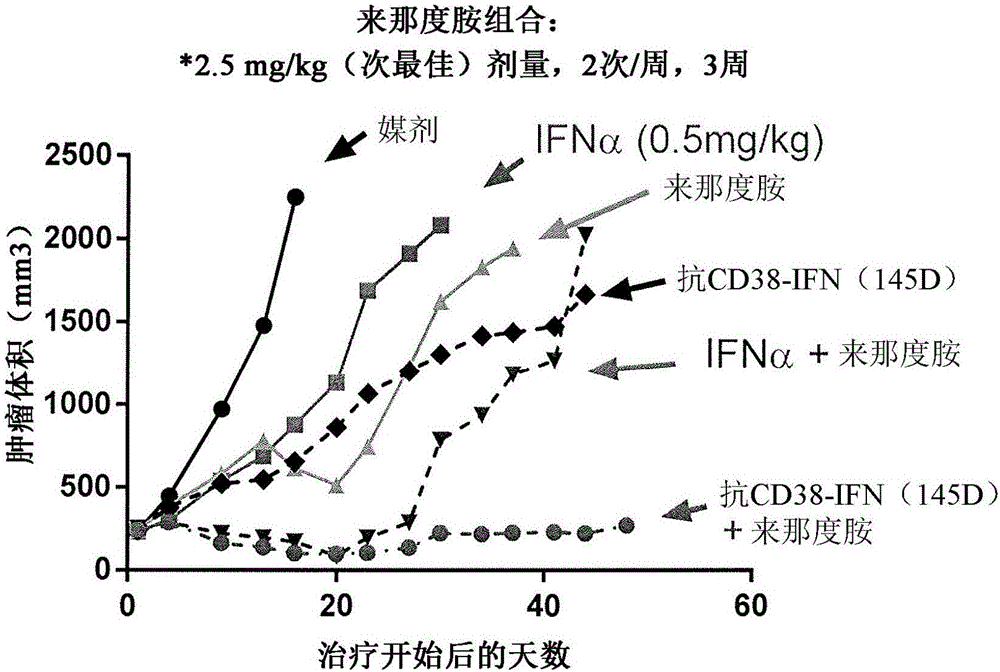
[0111] Cell line model of IFNα-2b construct + lenalidomide combination therapy inactivated by anti-CD38 antibody
[0112] In these experiments, 0.2 ml of 50% The matrix was implanted subcutaneously in the flank of 8-12 week old female CB.17 severe combined immunodeficiency (SCID) mice. When the tumor reaches 200-300mm 3When the average size of , mice were pair-matched into different groups and then treated with vehicle (PBS), 0.5 mg / kg of free non-sterilized interferon-α (IFN-α), a suboptimal dose of Anti-CD38 antibody-IFNα-2b-145D construct (2.5mg / kg molar equivalent equivalent to 0.5mg / kg IFN; intraperitoneally, biweekly as determined by the aforementioned in vivo efficacy study), isotype-matched antibody-IFNα - 2b-145D construct (isotype matched to anti-CD38 antibody, not anti-CD38 specific), lenalidomide alone (2.5mg / kg), free non-sterilized interferon alpha and lenalidomide combination of lenalidomide and suboptimal doses of anti-CD38 antibody-IFNα-2b-145D construct o...
example 2
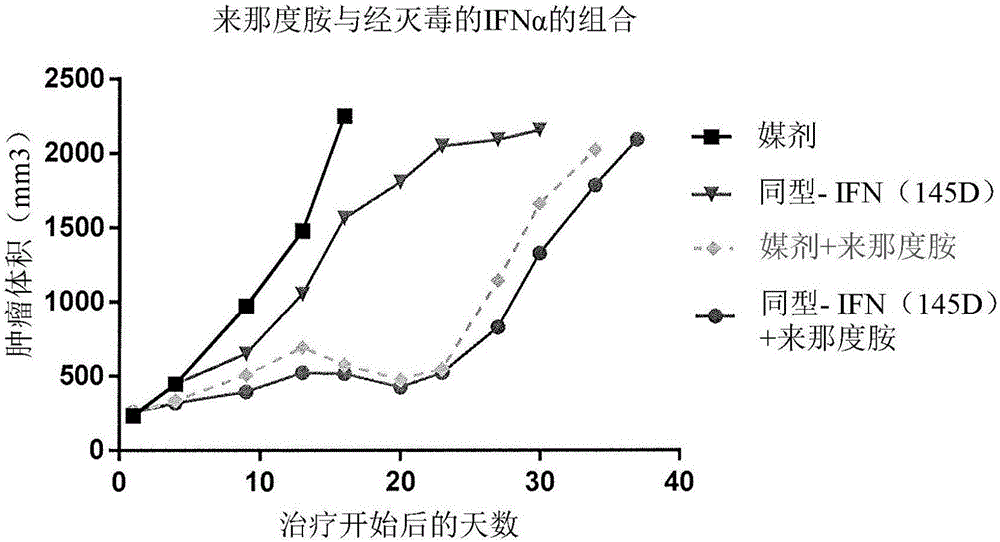
[0116] Cell line model of glycosylated IFNα-2b construct + lenalidomide combination therapy inactivated by anti-CD38 antibody
[0117] In this experiment, will contain 1×10 7 50% of H929 multiple myeloma cells 8-12 week old female CB.17 severe combined immunodeficiency (SCID) mice were implanted subcutaneously in the flank. Tumor volumes were measured biweekly by calipers. When the tumor reaches 170-350mm 3 At an average size of , mice were randomly assigned and treatment was initiated. If the tumor grows to more than 2000mm before completing the study by day 60 3 volume, then sacrifice the animal.
[0118] In this example, the dose levels and dosing intervals of the administration of a fusion of an anti-CD38 antibody and killed glycosylated interferon-α2b (A10.21(T106A)) in combination with lenalidomide were studied. A10.21(T106A) is an anti-CD38 IgG4 antibody x10.21 fused to glycosylated inactivated IFNα2b with substitutions A145D and T106A. Treatment regimens and re...
example 3
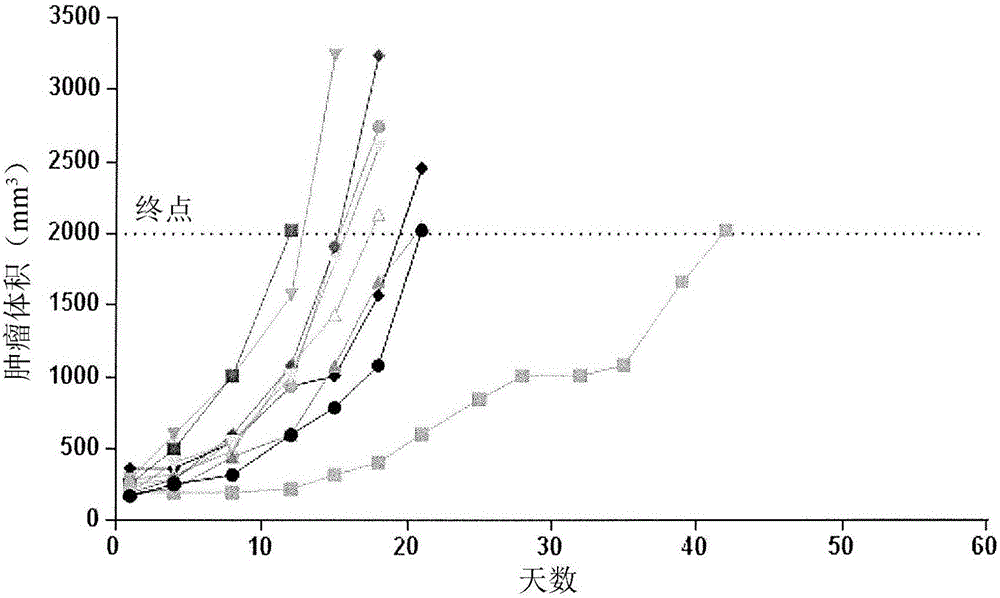
[0124] Pollidomide Research
[0125] These experiments were performed to determine the non-curative dose therapy of anti-CD38 antibody fused to sterilized interferon-α2b compared with the non-curative effect of polilidomide in the H929 human multiple myeloma xenograft model in female CB17SCID mice. Efficacy of combinations of curative dose therapies. Pollidomide, like lenalidomide, is a derivative and analog of thalidomide with increased efficacy and reduced toxicity against multiple myeloma.
[0126] Simply put, the 1×10 7 Sixty female CB.17 SCID mice were injected subcutaneously into the right flank of each H929 tumor cell. When the tumor reaches 150mm 3 Treatment with polilidomide and anti-CD38 antibody fused to sterilized interferon to α2b was initiated at the mean volume of . Study in tumor volume up to 2000mm 3 when terminated. The cohorts were divided as follows, as outlined in Table 6: Group 1, vehicle (PBS); Group 2, polilidomide alone (2.5 mg / kg); Group 3, IFNα...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com