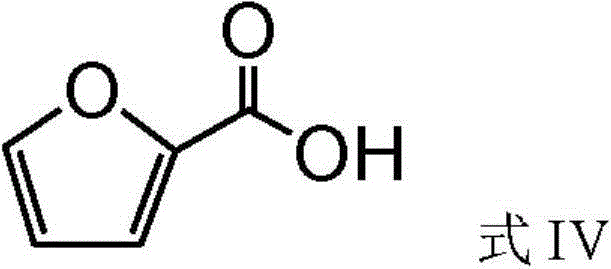
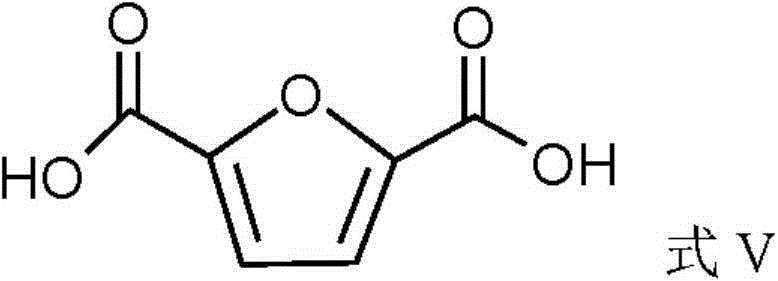
A method of preparing furan-2,5-dicarboxylic acid from furoic acid
A technology of furandicarboxylic acid and furan formic acid, which is applied in the direction of organic chemistry, can solve the problems of low total yield, high cost, and difficulty in large-scale industrial application, and achieve the goal of less by-products, short process, and promotion of sustainable development Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Take 11.2g of furoic acid monomer and put it into a 100ml reactor, add 20.4g of acetic anhydride, and react with 0.02mol concentrated sulfuric acid at 160°C for 2 hours. Base-5-furancarboxylic acid, yield 86%, liquid phase mass spectrometry (LC-MS) records molecular weight 154.0, 1 H-NMR (400MHz, deuterated chloroform CDCl3) test obtained, CH on the furan ring, 1H, δ (7.44); CH on the furan ring, 1H, δ (7.47); CH 3 , 3H, δ(2.59). Elemental analysis C7H6O4 calculated value C: 54.55, H: 3.92, O: 41.52; measured value C: 54.61, H: 4.10, O: 41.55.
[0061] In a 250ml reactor, dissolve 3.08g of 2-acetyl-5-furancarboxylic acid in 20ml of water, add 35.5g of iodine and 200ml of water dropwise at 20°C, then add NaOH aqueous solution (concentration is 2mol / L) dropwise to adjust the pH 7, the precipitate was removed by filtration, the pH value of the reaction solution was adjusted to 1 with hydrochloric acid (concentration: 0.5 mol / L), the precipitated solid was filtered and dr...
Embodiment 2
[0064] Take 11.2g of furoic acid monomer and put it into a 250ml reactor, add 102.6g of chloroacetic anhydride, and react with 0.025mol of concentrated nitric acid at 200°C for 0.5h. After the reaction is completed, cool down to room temperature, distill off chloroacetic anhydride under reduced pressure, and sublimate to obtain white crystals 2-Chloroacetyl-5-furancarboxylic acid, yield 93%, liquid phase mass spectrometry (LC-MS) measured molecular weight 188.6, 1 H-NMR (400MHz, CDCl 3 ) test, CH on the furan ring, 1H, δ (7.44); CH on the furan ring, 1H, δ (7.47); CH 2 , 2H, δ (4.83).
[0065] In a 250ml reactor, dissolve 3.77g of 2-chloroacetyl-5-furancarboxylic acid in 100ml of water, slowly feed 0.2mol of chlorine gas at 40°C, and at the same time add KOH aqueous solution (concentration: 1mol / L) dropwise, and then add chlorine gas Finish adjusting pH with KOH solution (concentration is 1mol / L) to be 7, filter to remove precipitate, use sulfuric acid (concentration is 0.5m...
Embodiment 3
[0068] Take 5.6g of furoic acid monomer and put it into a 250ml reactor, add 57.8g of trifluoroacetic anhydride, and react with 0.005mol concentrated hydrochloric acid at 160°C for 6h. Crystalline 2-trifluoroacetyl-5-furancarboxylic acid, yield 88%, molecular weight 207.0 as measured by liquid chromatography mass spectrometry (LC-MS), 1 H-NMR (400MHz, CDCl 3 ) test, CH on the furan ring, 1H, δ (7.74); CH on the furan ring, 1H, δ (7.97).
[0069] In a 250ml reactor, 4.14g of 2-trifluoroacetyl-5-furancarboxylic acid was dissolved in 20ml of water, 10.0g of bromine and 50ml of water were added at 60°C, and then LiOH aqueous solution (concentration was 2mol / L) was added dropwise, Adjust the pH to 7, remove the precipitate by filtration, adjust the pH of the reaction solution to 1 with phosphoric acid (concentration: 1 mol / L), filter the precipitated solid and dry it to obtain 2,5-furandicarboxylic acid with a yield of 70%.
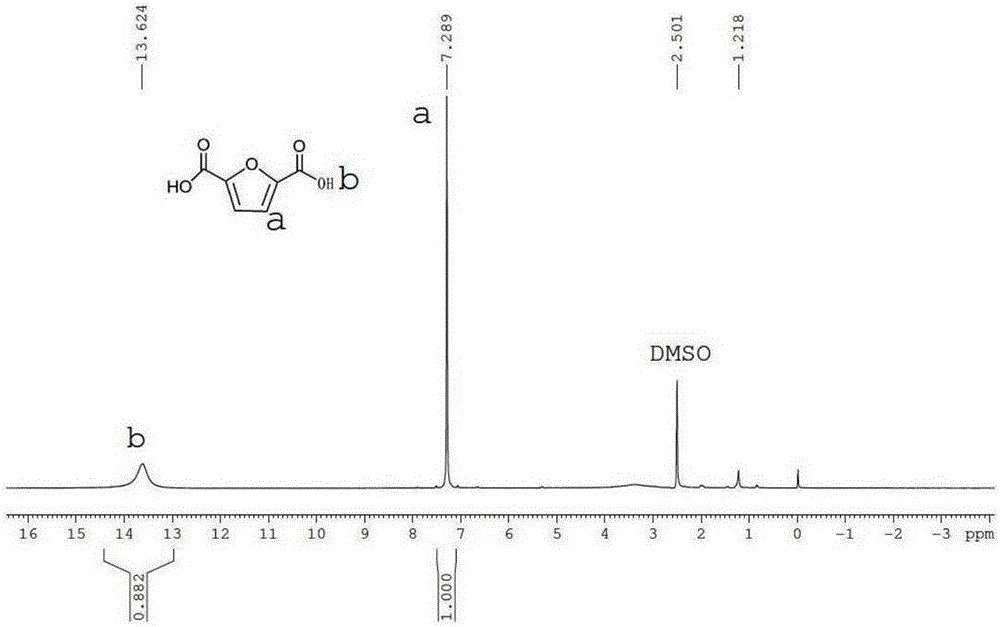
[0070] through 1 H-NMR (400MHz, DMSO) test, CH on the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com