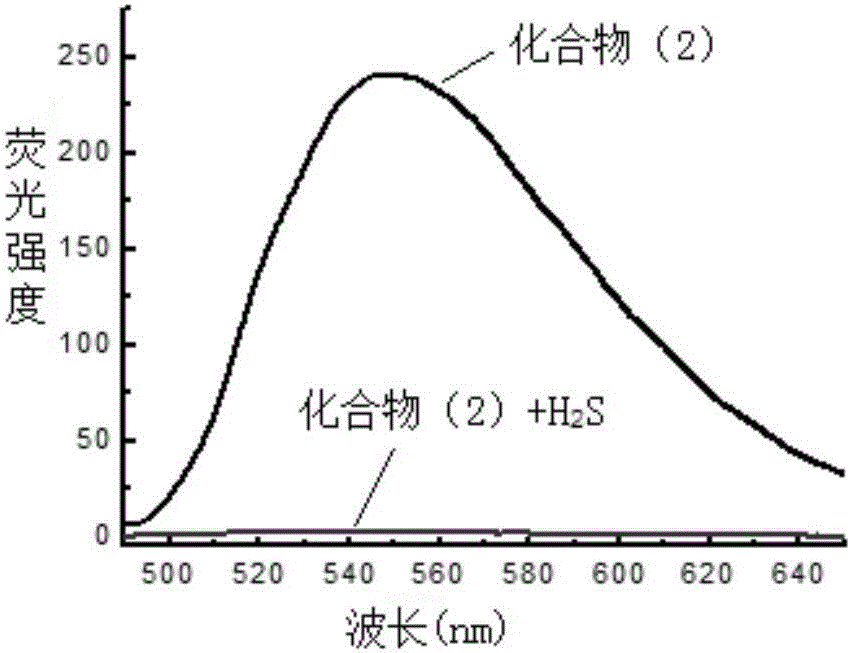
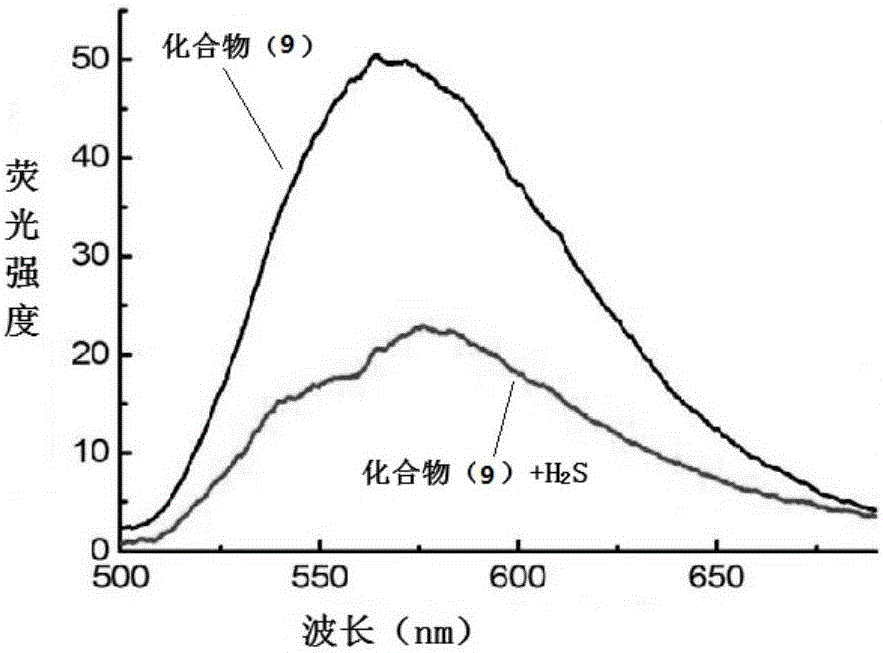
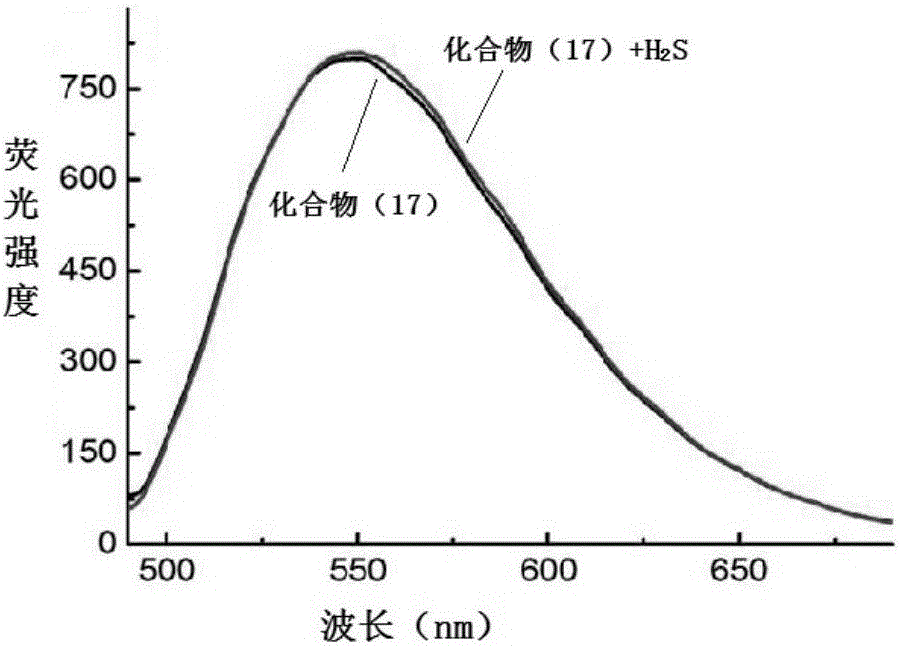
NBD-organic amine fluorescence probe, and preparation method and application thereof
A technology of fluorescent probes and organic amines, which is applied in the field of NBD-organic amine fluorescent probes and its preparation, which can solve the problems of inability to develop paper-based detection devices, color changes of colorimetric probes that cannot be seen by the naked eye in a short time, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Another aspect of the present invention provides a kind of preparation method of NBD-organic amine fluorescent probe, this method comprises:
[0035] (1) In the presence of the first organic solvent and a basic condensing agent, the compound shown in formula (15) is contacted with NBD-Cl to react; the first intermediate product is obtained;
[0036]
[0037] (2) In the presence of the second organic solvent, trifluoroacetic acid is contacted with the first intermediate product to obtain the compound shown in formula (16);
[0038]
[0039] (3) In the presence of a third organic solvent, contact the compound represented by formula (16) with acetic anhydride to obtain the NBD-organic amine fluorescent probe.
[0040] Preferably, the molar ratio of the compound represented by the formula (15) to NBD-Cl is 1.0-1.5:1; the molar ratio of the compound represented by the formula (16) to acetic anhydride is 1:1.5-2.
[0041] According to the present invention, the alkalin...
Embodiment 1
[0052] (1) Dissolve 1.2mmol mono-tBOC piperazine and 1.0mmol NBD-Cl in 20.0mL dichloromethane solvent, then add 0.25mL (1.5mmol) N,N-diisopropylethylamine (DIPEA); room temperature After stirring for 4 hours, distill under reduced pressure, and use flash column chromatography to purify the residue under reduced pressure. The eluent is methanol and dichloromethane with a volume ratio of 1.5:100 to obtain an intermediate product
[0053](2) In 10 mL of dichloromethane, contact 10 mL of trifluoroacetic acid with the intermediate product obtained in step (1), stir at room temperature for 3 h, and distill under reduced pressure to obtain a red solid
[0054] (3) In 20mL of dichloromethane, 1.0mmol of Contact reaction with 1.8mmol acetic anhydride, stir at room temperature for 3h, distill under reduced pressure, use flash column chromatography to purify the residue under reduced pressure, the eluent is methanol and dichloromethane with a volume ratio of 0.8:100, to obtain the o...
Embodiment 2
[0057] Dissolve 0.5mmol piperidine and 0.75mmol NBD-Cl in 10.0mL tetrahydrofuran; then add 0.25mL (1.5mmol) N,N-diisopropylethylamine, stir at room temperature for 1h, distill under reduced pressure, use flash column chromatography The vacuum distillation residue was purified by analytical method, and the eluent was petroleum ether and dichloromethane at a volume ratio of 1:2 to obtain the NBD-organic amine fluorescent probe compound formula (9).
[0058] Compound (9) is a red solid, TLC: Rf = 0.3 (petroleum ether: dichloromethane = 1:2).1H NMR (400MHz, CDCl3), δ: 8.41 (d, J = 8.8Hz, 1H), 6.27 ( d,J=8.8Hz,1H),4.14-4.07(m,4H),1.86-1.80(m,6H).13C NMR(100MHz,DMSO-d6),δ:145.08,144.92,144.65,136.29,119.95, 103.05, 50.95, 25.80, 23.40. HRMS (ESI): m / z [M+H] + calcd. for C11H13N4O3: 249.0988, found: 249.0979.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com