Application of PEDF in preparing medicine for diabetes bone tissue complication
A technology for bone tissue and complications, which is applied in the application field of preparing medicines for treating diabetic bone tissue complications, and can solve the problems of accelerating the osteogenesis process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Isolation and culture of osteoblasts
[0016] Bone waste samples were collected from foot amputee patients and divided into diabetic and non-diabetic groups. Tissue samples were provided by Penn State Hershey College of Medicine. After the collected tissue samples were rinsed with PBS for 3 times, the connective tissue and adipose tissue on the surface of the bone tissue were removed, and the cells were treated with alcohol for 15 seconds (to remove the fibroblasts on the surface of the bone tissue). Digest the bone tissue with collagenase at a concentration of 4 mg / ml, collect the supernatant of the digestion solution every 45 minutes, centrifuge at 1000 rpm for 5 minutes, and keep the precipitate for three consecutive times. After the fourth cell digestion for 45 minutes, collect the bone tissue and single cell pellet by centrifugation. Spread them evenly into 100mm cell culture dishes for culture. The composition of the culture medium is a-MEM, 10% FBS, 1...
Embodiment 2
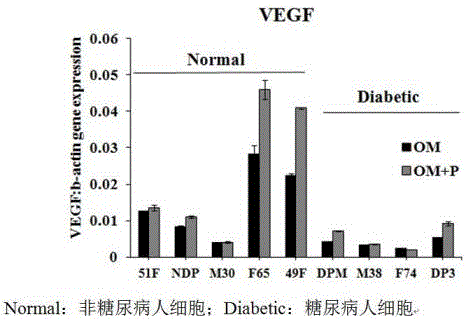
[0017] Example 2 PEDF up-regulates the level of VEGF in diabetic osteoblasts
[0018] According to the culture conditions of Example 1, 250ng / ml of PEDF was added to the osteogenic culture medium, and the level of VEGF was detected by conventional qPCR technology. The melting curve was 95°C 15s (100%); 65°C 1min, 95°C 15s (100%), +0.3°C / circle, b-actin was used as internal reference. The results showed that the expression of VEGF was up-regulated after the cells were treated with 250ng / ml PEDF for 7 days. like figure 2 shown.
Embodiment 3
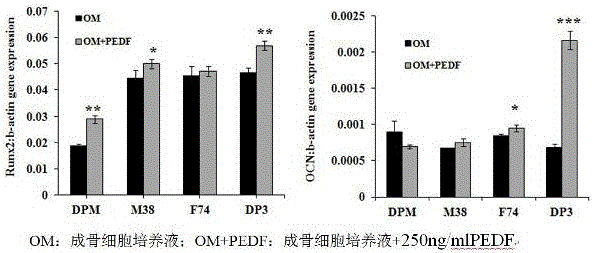
[0019] Example 3 PEDF up-regulates the expression levels of OCN and Runx2 genes
[0020] OCN and Runx2 are marker genes of osteoblasts. According to the culture conditions of Example 1, 250 ng / ml of PEDF was added to the osteogenic culture medium, and the expression levels of OCN and Runx2 genes were detected by conventional qPCR technology. ℃ 30s), the melting curve was 95℃ 15s (100%); 65℃ 1min, 95℃ 15s (100%), +0.3℃ / circle, b-actin was used as internal reference. The results showed that the expression of OCN and Runx2 genes was up-regulated after 250ng / ml PEDF treated the cells for 7 days. like image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com